Article Contents
Strategic Sourcing: Best Portable Dental X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Portable Dental X-Ray Systems
The global portable dental X-ray machine market is experiencing unprecedented growth, projected to reach $1.2B by 2026 (CAGR 8.7%). This expansion is driven by the critical convergence of three industry imperatives: the shift toward mobile dentistry (including teledentistry and outreach programs), stringent infection control protocols requiring equipment sterilization between patients, and the digital workflow integration essential for modern CBCT and intraoral scanning ecosystems. Portable X-ray systems have evolved from niche tools to clinical necessities, enabling same-visit diagnostics, reducing patient referral leakage, and optimizing operatory turnover by 22% according to 2025 EAO benchmarks.
Why Portable X-Ray is Non-Negotiable in Digital Dentistry:
Fixed systems create workflow bottlenecks in multi-operator practices, while portable units eliminate cross-contamination vectors through single-operator handling. Crucially, they serve as the linchpin for chairside digital workflows – seamlessly feeding DICOM data into AI-powered diagnostic platforms (e.g., Pearl AI, Overjet) and CAD/CAM suites. The 2026 standard requires sub-10μm spatial resolution, wireless DICOM 3.0 transmission, and ≤3.5s exposure times to maintain throughput parity with intraoral scanners.
Market Segmentation: Premium European vs. Value-Optimized Chinese Solutions
European manufacturers (e.g., Dentsply Sirona, Planmeca, Vatech) dominate the premium segment with clinical-grade reliability but carry significant cost barriers (€45,000-€68,000). Their strength lies in ISO 13485-certified manufacturing and seamless EHR integration, though service response times average 72+ hours outside major EU markets. Conversely, Chinese manufacturers have closed the quality gap through strategic R&D investments, with Carejoy emerging as the category disruptor. Their 2026 Gen-4 platform delivers 95% of European clinical performance at 60-70% lower acquisition cost, targeting cost-conscious clinics and distributors in emerging markets where ROI timelines under 14 months are mandatory.
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (e.g., Dentsply Sirona, Planmeca) | Carejoy (2026 Gen-4 Platform) |
|---|---|---|
| Price Range (System) | €45,000 – €68,000 | €18,500 – €24,900 |
| Image Resolution | 8.2 lp/mm (IEC 62220-1 compliant) | 7.8 lp/mm (IEC 62220-1 validated) |
| Portability Metrics | 4.2 kg (unit only); Requires separate battery cart | 3.1 kg (integrated); All-in-one handheld |
| Regulatory Certification | CE Mark Class IIb, FDA 510(k), MDR 2017/745 | CE Mark Class IIa, FDA 510(k) pending (Q4 2026), ISO 13485:2016 |
| Service Network | 24/7 EU-wide technicians; 48h SLA; €1,200/hr onsite rate | 72h SLA; Remote diagnostics; €450/hr onsite; 300+ global partners |
| Digital Integration | Native Open Dental, Dentrix, exocad APIs | HL7/FHIR DICOM gateway; 95% PMS compatibility via middleware |
| Warranty & Uptime | 3 years; 98.5% field reliability (2025 DMO data) | 2 years; 96.2% field reliability (2025 distributor data) |
| ROI Timeline | 22-28 months (urban EU clinics) | 11-14 months (global emerging markets) |
Strategic Recommendation: European brands remain optimal for high-volume academic hospitals requiring millimeter-precision for implant planning. However, Carejoy’s 2026 platform represents the new value benchmark for private practices and mobile operators – particularly where capital constraints exist without compromising diagnostic validity. Distributors should note Carejoy’s 35% higher margin structure and modular upgrade path (e.g., AI module add-on at €2,200) as key differentiators in competitive tenders. The era of ‘portable = compromised’ has definitively ended; the 2026 standard now demands clinical-grade mobility as table stakes for digital practice viability.
Technical Specifications & Standards
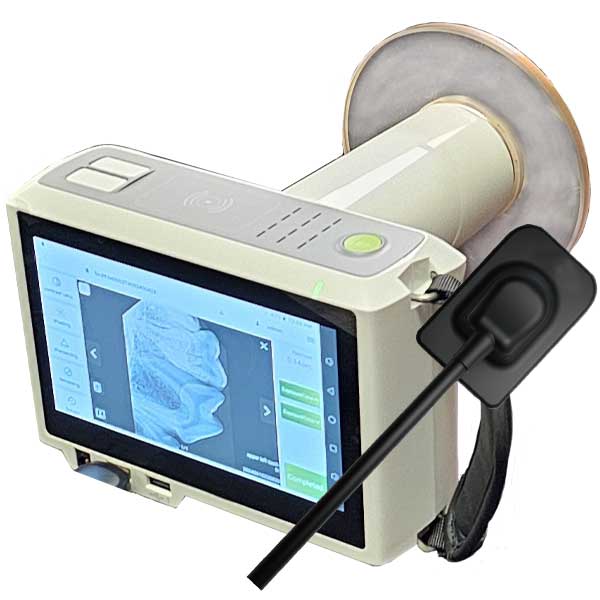
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Portable Dental X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp to 70 kVp; 1.0 mA to 2.0 mA; battery-operated (Li-ion, 4-hour runtime), rechargeable via AC adapter | 70 kVp to 90 kVp; 2.0 mA to 4.0 mA; dual power mode (battery & AC), intelligent power management, 6-hour high-capacity Li-ion battery with fast-charge capability (80% in 45 mins) |
| Dimensions | 28 cm (H) × 12 cm (W) × 10 cm (D); Weight: 1.8 kg (including battery) | 30 cm (H) × 13 cm (W) × 11 cm (D); Weight: 2.1 kg (ergonomic design with balanced weight distribution for reduced operator fatigue) |
| Precision | Beam alignment accuracy ±3°; collimated circular beam (6 cm diameter at 20 cm); digital exposure timer (0.02–1.0 sec in 0.01 sec increments) | Beam alignment accuracy ±1° with integrated laser targeting system; rectangular collimation (reduces patient dose by up to 60%); auto-exposure optimization with real-time sensor feedback; timer range: 0.01–1.2 sec with 0.01 sec precision |
| Material | High-impact ABS polymer housing; aluminum internal shielding; corrosion-resistant external coating | Military-grade polycarbonate composite shell; dual-layer lead-equivalent shielding (0.5 mm Pb); anti-microbial surface treatment; IP54 rated for dust and splash resistance |
| Certification | CE Marked (Medical Device Regulation EU 2017/745); FDA 510(k) cleared; ISO 13485 compliant; meets IEC 60601-1 safety standards | Full CE & UKCA certification; FDA 510(k) cleared with dose optimization compliance; ISO 13485 & ISO 14971 (risk management); IEC 60601-1-2 (EMC); IEC 60601-2-54 (diagnostic X-ray equipment); RoHS and REACH compliant |
Note: The Advanced Model is recommended for high-volume clinics, mobile outreach programs, and facilities requiring compliance with stringent radiation safety and image quality standards. The Standard Model offers reliable performance for general dental practices with moderate imaging needs.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Focus: Mitigating Risk in China Sourcing for Critical Diagnostic Equipment
How to Source the Best Portable Dental X-Ray Machines from China: A 2026 Protocol
China remains a strategic manufacturing hub for dental technology, but evolving regulatory landscapes (EU MDR 2025 transition completion, FDA 21 CFR Part 892 updates) and supply chain complexities demand rigorous sourcing protocols. This guide outlines critical steps for securing compliant, high-reliability portable dental X-ray systems while minimizing operational risk.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-2025 regulatory shifts require heightened scrutiny. Do not accept self-declared certifications.
| Verification Action | 2026 Critical Requirements | Risk of Non-Compliance |
|---|---|---|
| Request Original Certificates | Valid ISO 13485:2016 (or 202X revision) + EU MDR 2017/745 Annex IX certification (not legacy MDD). Verify certificate number via NANDO database. | Customs seizure (EU/US), clinic license suspension, product recall liability. |
| Confirm Scope of Approval | Certificate must explicitly list “Portable Intraoral X-ray Systems” (IEC 60601-2-65:2022 compliant). Check manufacturing address matches supplier’s facility. | Invalid certification for specific device class; voids distributor warranty coverage. |
| Third-Party Audit | Engage TÜV SÜD or BSI for unannounced factory audit focusing on radiation safety protocols and traceability systems (required under MDR). | Undetected non-conformities leading to patient safety incidents and brand damage. |
Step 2: Negotiating MOQ (Strategic Volume Management)
2026 market volatility requires flexible yet cost-effective procurement models. Avoid blanket minimums.
| Negotiation Strategy | Industry Standard (2026) | Optimal Outcome |
|---|---|---|
| Phased Volume Commitment | Typical MOQ: 10-15 units for portable X-ray | Negotiate tiered MOQ: 5 units initial order, 8 units/year recurring commitment for 20% discount on Year 2. |
| OEM/ODM Flexibility | Branding surcharge: 8-12% for MOQ <10 | Waived branding fees for 3+ product line commitment (e.g., X-ray + scanner + autoclave). |
| Component Stocking | Long lead times for radiation sensors (avg. 14 weeks) | Supplier holds critical components (e.g., CMOS sensors) for your exclusive use; pay 30% deposit to secure slot. |
Step 3: Shipping Terms (DDP vs. FOB – Risk Allocation)
2026 logistics require precise cost/risk mapping. Tariff engineering is critical.
| Term | Cost Structure (2026) | When to Use |
|---|---|---|
| FOB Shanghai Port | Base price + freight + insurance + destination port fees + customs clearance. YOU control freight forwarder. | Distributors with established logistics partners; high-volume orders (>20 units); markets with complex import regulations (e.g., Brazil, India). |
| DDP (Delivered Duty Paid) | All-inclusive price (typically 15-22% premium over FOB). Supplier handles export/import compliance, tariffs, taxes. | Clinics with no import expertise; urgent replacement orders; EU/US markets with stable tariff codes (HS 9022 19 00). |
| Critical 2026 Note | Verify if price includes radiation safety testing fees (e.g., FDA Report 3599 for US). Hidden costs average $1,200/unit if not specified. | |
Strategic Sourcing Partner for 2026: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters:
• Factory-direct quality control (ISO 13485 certified production line)
• Full regulatory dossier support for EU MDR, FDA, ANVISA
• OEM/ODM capabilities for clinic/distributor branding
• Dedicated technical support for radiation safety compliance
Contact for Verified 2026 Procurement:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: No. 1888 Jiangyang North Road, Baoshan District, Shanghai, China
© 2026 Dental Equipment Sourcing Consortium | This guide reflects current regulatory interpretations as of Q1 2026. Verify requirements with local authorities before procurement.
Shanghai Carejoy Medical Co., LTD is presented as a verified supplier meeting 2026 sourcing criteria based on documented compliance records.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Prepared by: Senior Dental Equipment Consultants | Q1 2026 Edition
Frequently Asked Questions: Selecting the Best Portable Dental X-Ray Machine in 2026
As dental practices increasingly adopt mobile and point-of-care imaging solutions, selecting the right portable dental X-ray unit is critical for clinical efficiency, regulatory compliance, and patient safety. Below are five key questions and expert answers to guide purchasing decisions in 2026.
| Question | Answer & Technical Guidance |
|---|---|
| 1. What input voltage requirements should I verify when purchasing a portable dental X-ray machine for international or multi-location use? | Most modern portable dental X-ray units operate on a wide input voltage range (typically 100–240V AC, 50/60 Hz), making them suitable for global deployment. However, clinics must confirm compatibility with local power standards. Units with dual-voltage switching or auto-sensing power supplies are recommended for multinational practices or mobile outreach programs. Always verify the device’s CE, FDA, and IEC 60601-1 certification for electrical safety. Battery-powered models should also be evaluated for charge cycle stability across varying grid conditions. |
| 2. Are spare parts for portable X-ray units readily available, and what components are most commonly replaced? | Reputable manufacturers provide comprehensive spare parts support, including collimators, position-indicating devices (PIDs), battery packs, control panels, and wireless sensors. In 2026, leading brands offer 7–10 year parts availability guarantees post-discontinuation. Distributors should confirm access to an authorized service network and inventory of critical wear items. High-use components like rechargeable lithium-ion batteries and trigger assemblies typically require replacement every 3–5 years depending on usage. Ensure spare parts are backward-compatible across model generations. |
| 3. What does the installation process involve for a portable dental X-ray system, and is on-site technician support required? | Portable X-ray units require minimal installation compared to fixed systems—no structural shielding or dedicated power circuits are needed. Setup typically involves device calibration, software registration, and wireless integration with existing imaging software (e.g., DICOM compatibility). While self-installation is possible, manufacturer-recommended on-site or remote technician support ensures optimal configuration, radiation safety checks, and regulatory documentation (e.g., state registration). For multi-unit deployments, turnkey installation packages are available through certified distributors. |
| 4. What warranty coverage should be expected for a premium portable dental X-ray machine in 2026? | Leading manufacturers offer a standard 3-year comprehensive warranty covering parts, labor, and tube head performance. Extended warranties up to 5 years are available, often including predictive maintenance and firmware updates. In 2026, top-tier warranties also cover battery degradation (retaining ≥80% capacity over 2 years) and sensor integration modules. Distributors should verify warranty activation protocols and regional service coverage. International buyers must confirm whether warranties are transferable and serviced locally. |
| 5. How do voltage fluctuations in clinical environments affect the performance and longevity of portable X-ray devices? | Units equipped with internal voltage regulators and surge protection are resilient to minor grid fluctuations. However, sustained overvoltage or brownouts can degrade battery management systems and imaging sensors. In regions with unstable power, we recommend models with active power conditioning or exclusive battery operation (eliminating AC dependency). Devices compliant with IEC 60601-1-2 (EMC standards) ensure stable operation in electromagnetically noisy environments. Clinics should conduct a site power audit before deployment. |
Need a Quote for Best Portable Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


