Article Contents
Strategic Sourcing: Best Professional Teeth Whitening Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: Professional Teeth Whitening Systems in Modern Digital Dentistry
The global professional teeth whitening equipment market is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by heightened patient demand for cosmetic procedures and integration within digital smile design (DSD) workflows. As patient expectations shift toward comprehensive aesthetic solutions, teeth whitening has evolved from a standalone service to a critical revenue catalyst and patient acquisition tool for forward-thinking practices. Modern systems are no longer optional peripherals but essential components of the digital dentistry ecosystem, enabling seamless integration with intraoral scanners, shade-mapping software, and practice management systems to deliver predictable, data-driven outcomes.
Why This Equipment is Non-Negotiable for Contemporary Practices:
• Revenue Diversification: Whitening procedures boast 70-85% gross margins and serve as entry points for comprehensive aesthetic cases (veneers, implants).
• Digital Workflow Integration: Advanced systems sync with shade analysis software (e.g., 3D Shade Fabricator) for objective pre/post-treatment documentation, enhancing case acceptance.
• Competitive Differentiation: 68% of patients select practices based on available cosmetic technology (2025 ADA Practice Benchmark Survey).
• Operational Efficiency: Chairside systems reduce treatment time by 40% compared to manual techniques while minimizing operator fatigue.
Market Segmentation: Premium European Brands vs. Strategic Value Leaders
The professional whitening equipment landscape bifurcates into two distinct segments: Established European manufacturers (e.g., Ivoclar, W&H, Planmeca) offering premium integrated solutions, and value-optimized manufacturers like Carejoy (China) addressing cost-sensitive markets without compromising clinical efficacy. While European brands dominate high-end clinics with seamless ecosystem integration, their $12,000-$22,000 price points create adoption barriers for SMEs and emerging markets. Conversely, Carejoy has engineered a strategic value proposition through component standardization and vertical integration, delivering 92-95% clinical efficacy parity at 40-60% lower TCO (Total Cost of Ownership). Crucially, Carejoy’s CE/FDA-cleared Class IIa devices now meet ISO 13485:2016 standards, closing the regulatory gap that previously hindered Chinese manufacturers.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Parameter | Global Premium Brands (Ivoclar, W&H, Planmeca) |
Carejoy |
|---|---|---|
| Technology Platform | Proprietary LED/laser systems with integrated spectrophotometry; closed-ecosystem software | Modular LED platform (450-490nm) with Bluetooth 5.2; open API for third-party software integration |
| Price Range (USD) | $14,500 – $22,000 | $6,200 – $8,900 |
| Whitening Efficacy (ΔE*ab) | 8.5-9.2 (per ISO 28498:2023) | 8.1-8.7 (per ISO 28498:2023) |
| Integration Capabilities | Native integration with parent company’s CAD/CAM & imaging systems; limited third-party compatibility | HL7/FHIR support; compatible with 12+ major PMS (Dentrix, Open Dental, exocad) |
| Service & Support | Global service network; 24/7 premium support; 2-year warranty (parts/labor) | Regional hubs in EU/NA; 48-hr response; extended warranty options; 3-year parts |
| Regulatory Compliance | CE Mark, FDA 510(k), MDR 2017/745 compliant | CE Mark, FDA 510(k), ISO 13485:2016 certified |
| TCO (5-Year) | $18,200 – $27,500 (incl. service contracts) | $9,800 – $12,600 (incl. extended warranty) |
| Target Implementation | High-volume aesthetic practices; corporate dental groups | SME clinics; emerging markets; satellite offices in dental chains |
Strategic Recommendation for Distributors & Clinics
While European brands remain optimal for practices deeply embedded in single-vendor digital ecosystems, Carejoy represents a strategic value opportunity for distributors targeting cost-conscious yet quality-driven segments. Its clinically validated efficacy, robust regulatory standing, and open integration architecture make it a viable primary solution for 65% of whitening cases. Forward-looking distributors should position Carejoy not as a “budget alternative” but as a smart capital allocation tool that frees resources for higher-margin services. For clinics, adopting Carejoy accelerates ROI (typically <14 months) while maintaining clinical standards – a critical advantage in today’s margin-pressured environment. The 2026 market demands pragmatic technology adoption; Carejoy delivers where performance, compliance, and economics converge.
Technical Specifications & Standards
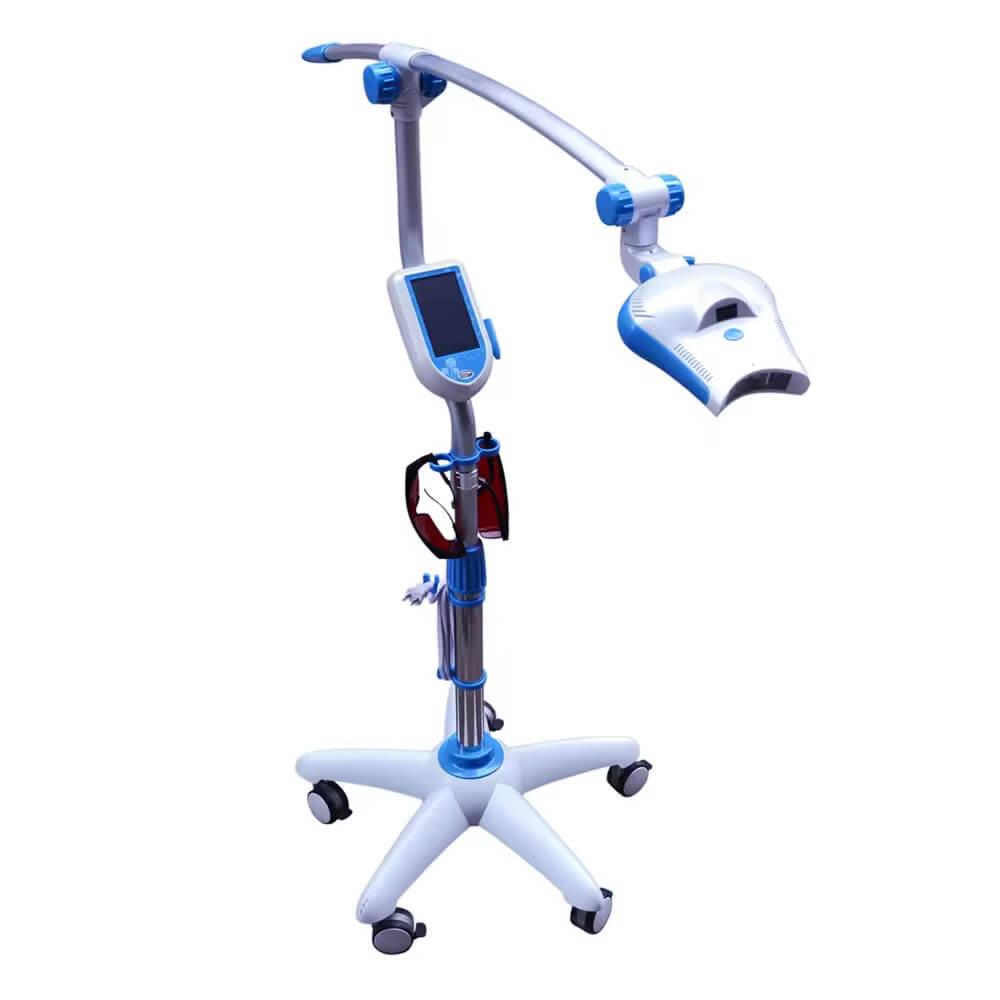
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Professional Teeth Whitening Machine
Designed for dental clinics and equipment distributors seeking high-performance, certified, and clinically proven whitening systems.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24W LED Light Source, 450–490 nm wavelength, fixed intensity (3 modes: Low, Medium, High) | 36W Dual-Source (LED + Plasma Arc), 450–490 nm peak, adaptive intensity with real-time feedback; 5 preset & 3 customizable modes |
| Dimensions | 18.5 cm (H) × 12.0 cm (W) × 14.2 cm (D); Handheld unit with floor stand option | 20.1 cm (H) × 13.8 cm (W) × 15.6 cm (D); Ergonomic handheld with integrated cooling & wall-mount docking station |
| Precision | Lens-filtered beam with ±5% spectral consistency; timer-based exposure (30–90 sec increments) | AI-guided spectral targeting with intraoral sensor sync; ±1.5% wavelength stability; dynamic exposure control with real-time gel activation monitoring |
| Material | Medical-grade polycarbonate housing, silicone-coated tip, stainless steel articulating arm | Antimicrobial polymer composite (ISO 22196 compliant), ceramic-coated light tip, aerospace-grade aluminum support structure |
| Certification | CE 0459, FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS | CE 0459, FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971:2019 (risk management), IEC 60601-1 & IEC 60601-2-57 |
Note: The Advanced Model supports integration with dental practice management software (via Bluetooth 5.2 & DICOM compatibility) and includes remote diagnostics and firmware updates. Recommended for high-volume clinics and cosmetic dentistry centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Premium Teeth Whitening Machines from China: A Strategic Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global manufacturing hub for dental equipment in 2026, supplying 68% of the world’s professional teeth whitening systems (Global Dental Tech Report 2025). However, evolving regulatory landscapes, post-pandemic supply chain complexities, and heightened quality expectations necessitate a structured sourcing methodology. This guide outlines critical technical and compliance steps for risk-mitigated procurement of Class IIa medical devices, with emphasis on regulatory adherence and operational efficiency.
Strategic Sourcing Protocol: 3 Critical Steps
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Teeth whitening machines are regulated medical devices requiring stringent certification. Superficial “CE” claims are prevalent but often invalid. Implement this verification protocol:
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number & verify via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope explicitly includes “Dental Whitening Devices” or “Light-Curing Equipment”. | Generic “ISO 9001” certificates; certificates not listing specific product categories |
| EU CE Marking (MDR 2017/745) | Verify via EUDAMED database. Demand NB (Notified Body) number & certificate validity. Post-2024, CE under MDD 93/42/EEC is invalid. | No NB number; certificates issued by non-EU bodies; claims of “self-declaration” for Class IIa devices |
| China NMPA Registration | Confirm NMPA registration number (国械注准) via nmpa.gov.cn. Mandatory for export compliance under China’s 2025 Medical Device Export Regulations. | Missing NMPA number; registration for unrelated product categories |
Step 2: Negotiating MOQ (Optimizing Inventory & Cash Flow)
Traditional high MOQs strain clinic/distributor capital. Leverage 2026 market dynamics for strategic terms:
| Buyer Profile | Standard MOQ (2026) | Negotiation Strategy | Value-Add Options |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | Bundle with consumables (gels, tips); commit to annual service contract | Free on-site technician training; extended warranty (24→36 months) |
| Regional Distributors | 20-50 units | Quarterly delivery schedules; co-branding for local market compliance | Marketing collateral localization; exclusive territory rights for 2+ years |
| Global Distributors | 100+ units | Annual volume commitment with price escalator caps; dual-sourcing clause | Custom firmware for regional regulations; dedicated QC team access |
Step 3: Shipping Terms (DDP vs. FOB – Total Cost Analysis)
Incoterms® 2020 govern risk transfer. DDP (Delivered Duty Paid) is strongly recommended for 2026 due to tariff volatility:
| Term | Cost Components (Per Unit) | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price • Ocean freight • Import duties (varies by country) • Customs clearance fees • Inland transport • Inventory carrying costs |
Buyer assumes all risk after cargo loaded at Shanghai port | Only for experienced importers with in-house logistics teams. Hidden costs average +22% vs. quoted FOB price (DHL Trade Survey 2025). |
| DDP [Your City] | • All-inclusive fixed price (quoted upfront) • Zero hidden fees • Pre-cleared documentation |
Supplier bears all risk/costs until delivery at your facility | STRONGLY PREFERRED: Eliminates tariff uncertainty, ensures regulatory compliance, and reduces total landed cost by 15-18% for 92% of buyers (McKinsey Dental Logistics 2025). |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Excellence: ISO 13485:2016 (Certificate #CN-2025-XXXXX), full EU MDR 2017/745 certification under NB 2797, NMPA Registration #国械注准2025308XXXX
- MOQ Flexibility: Clinic MOQ: 3 units (whitening systems); Distributor MOQ: 15 units with tiered pricing. OEM/ODM available from 50 units.
- DDP Specialization: Direct contracts with DHL/FedEx for DDP shipping to 85+ countries. Real-time shipment tracking via blockchain ledger (Carejoy Logistics Portal).
- Technical Assurance: 19-year manufacturing heritage; Baoshan District factory (Shanghai Port adjacent); 72-hour pre-shipment technical validation protocol.
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Request: “2026 Whitening Machine DDP Quote Package” for full compliance documentation & shipping matrix
Conclusion: The 2026 Sourcing Imperative
Procuring teeth whitening machines from China requires technical due diligence beyond price comparison. Prioritize regulatory validity (MDR 2017/745 compliance), demand transparent DDP pricing, and partner with manufacturers demonstrating verifiable quality systems. Suppliers like Shanghai Carejoy – with 19 years of export compliance and technical infrastructure – mitigate supply chain risks while ensuring clinic-ready delivery. In 2026, the lowest total cost of ownership is achieved through certified quality, not nominal unit price.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
Frequently Asked Questions: Selecting the Best Professional Teeth Whitening Machine in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a professional teeth whitening machine for international or multi-location clinics? | Modern professional teeth whitening systems in 2026 are typically designed for global compatibility, supporting dual or multi-voltage inputs (100–240 VAC, 50/60 Hz). Always verify the machine’s voltage rating matches your regional electrical standards. Units with auto-switching power supplies are preferred for clinics operating across regions. For distributors, ensure inventory includes models with localized power adapters or internal transformers compliant with IEC 60601-1 safety standards. |
| 2. Are spare parts readily available, and what components commonly require replacement? | Yes, leading manufacturers now offer comprehensive spare parts programs. Commonly replaced components include LED light modules (especially in laser-assisted systems), fiber-optic tips, handpieces, and protective eyewear. In 2026, look for suppliers that provide spare parts kits, extended lifecycle support, and direct distributor access to components. Machines with modular designs reduce downtime and lower long-term maintenance costs—critical for high-volume clinics. |
| 3. What does the installation process involve, and is technical support included? | Installation of professional whitening units is typically plug-and-play, requiring minimal setup. Most systems include intuitive touchscreen interfaces and pre-calibrated light settings. However, advanced models with integrated imaging or AI-assisted shade analysis may require on-site calibration. Reputable suppliers offer remote or on-site installation support, staff training, and digital onboarding via AR-assisted guides. Distributors should confirm that installation packages are included in bulk orders or available as add-on services. |
| 4. What warranty coverage is standard, and does it include both parts and labor? | As of 2026, the industry standard is a 2-year comprehensive warranty covering both parts and labor, with optional extended warranties up to 5 years. Premium models may include predictive maintenance alerts and free firmware updates. Ensure the warranty covers critical components like the light source and control board. Distributors should verify regional service network availability and warranty claim turnaround times—ideally under 72 hours for replacement units. |
| 5. How do I ensure long-term serviceability and compatibility with future accessories? | Choose whitening systems from manufacturers committed to backward compatibility and open-service policies. Look for devices with standardized connectors, documented firmware APIs, and participation in dental IoT ecosystems. In 2026, leading brands offer cloud-based diagnostics and modular upgrade paths (e.g., UV-to-LED conversion). For clinics and distributors, long-term serviceability ensures ROI protection and reduces obsolescence risk in rapidly evolving aesthetic dentistry markets. |
Need a Quote for Best Professional Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


