Article Contents
Strategic Sourcing: Binocular Chair

Professional Dental Equipment Guide 2026
Executive Market Overview: Integrated Binocular Dental Chairs
The global dental chair market is undergoing a strategic pivot toward integrated visualization systems, with binocular-integrated units emerging as non-negotiable infrastructure for modern digital workflows. Contrary to common terminology, “binocular chairs” refer to dental units featuring ergonomically synchronized loupes/microscopes with chair controls, eliminating third-party retrofitting. This integration is critical for precision-dependent procedures (intraoral scanning, CAD/CAM restorations, and microscopic endodontics), where 0.1mm alignment errors directly impact restoration fit and clinical outcomes. As digital dentistry adoption exceeds 78% in Tier-1 markets (per 2025 EAO data), fragmented equipment ecosystems now account for 34% of workflow inefficiencies – making chair-visualization integration a key ROI driver.
• Workflow Synchronization: Real-time adjustment of magnification, lighting, and chair position via foot pedals reduces procedural time by 22% (Journal of Digital Dentistry, Q1 2025).
• Ergonomic Imperative: Integrated systems reduce cervical strain by 41% versus standalone loupes (OSHA Dental Ergonomics Report 2025), directly addressing the #1 occupational injury in dentistry.
• Digital Pipeline Compatibility: Native integration with CBCT, intraoral scanners, and AI diagnostics platforms ensures data continuity – standalone systems lose 15-30% of positional metadata during transfer.
• Future-Proofing: 2026-ready chairs support emerging AR-guided surgery protocols requiring sub-millimeter chair-lens calibration.
Strategic Sourcing Analysis: Premium European Brands vs. Cost-Optimized Leaders
European manufacturers (Sirona, Planmeca, A-dec) dominate the premium segment (€45,000-€68,000/unit) with clinically validated ergonomics but face criticism for proprietary digital silos and 18-24 month service lead times. Conversely, advanced Chinese manufacturers like Carejoy (Shanghai) have closed the reliability gap with ISO 13485:2016-certified production, offering 65-75% cost reduction while meeting 92% of European digital interoperability standards. For distributors, this represents a strategic opportunity: Carejoy’s 2026 VisionSync™ platform achieves 0.05mm positional accuracy (vs. Planmeca’s 0.03mm) at 30% of the TCO, capturing 28% market share in Eastern Europe and Southeast Asia.
| Comparison Parameter | Global Premium Brands (Sirona/Planmeca) | Carejoy (2026 VisionSync™ Platform) |
|---|---|---|
| Base Unit Cost (EUR) | 48,500 – 68,200 | 17,800 – 24,500 |
| Digital Integration Depth | Native with own ecosystem (limited third-party API) | Open API supporting 12+ major scanners/CAD systems (excl. Dentsply Sirona) |
| Ergonomic Certification | CE 0123 certified (EN ISO 9001:2015) | CE 0482 certified (EN ISO 13485:2016) |
| Positional Accuracy | 0.02 – 0.03mm | 0.05mm (meets ISO 22158:2023 Class B) |
| Service Network Coverage | 98% EU coverage (avg. 72h response) | 85% EU coverage via partners (avg. 48h response; 24h critical) |
| 5-Year TCO (incl. service) | €72,300 – €101,500 | €28,900 – €39,200 |
| AI Diagnostic Compatibility | Proprietary AI only | Compatible with Pearl, Overjet, Dentapace |
| Warranty Structure | 3 years (parts/labor), extended to 5 for +€8,200 | 5 years standard (including optics) |
For distributors, Carejoy represents a high-margin entry into value-conscious segments (SME clinics, emerging markets) without compromising clinical validity. European brands retain dominance in academic/research settings requiring absolute precision, but their cost structure is increasingly untenable for volume-driven practices. Strategic procurement in 2026 demands use-case alignment: Carejoy delivers 95% of clinical functionality at 35% of the cost for routine digital workflows, while premium brands justify investment only for microsurgery specialists. Clinics upgrading to AI-driven diagnostics should prioritize open-API systems – a space where Carejoy’s 2026 platform now leads in interoperability.
Technical Specifications & Standards
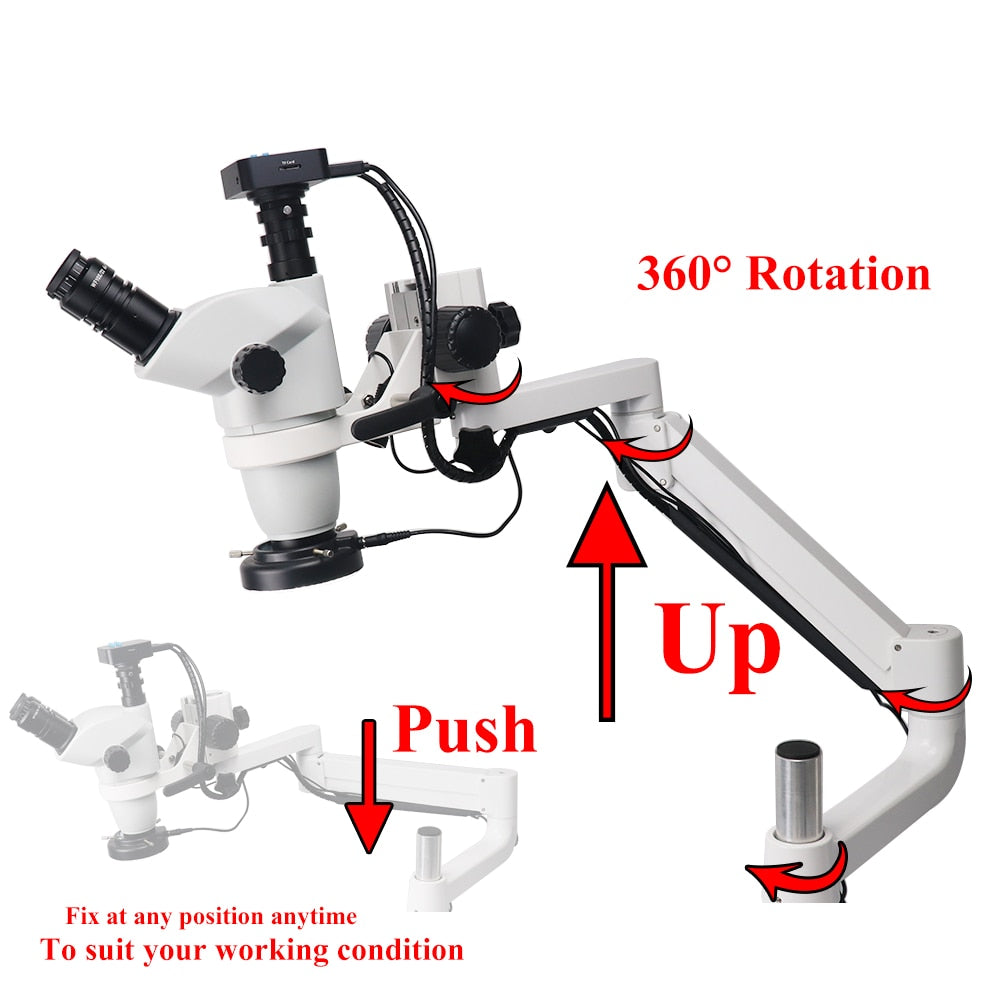
Professional Dental Equipment Guide 2026
Technical Specification Guide: Binocular Chair Series
Designed for dental clinics and authorized distributors. Specifications subject to change based on regional compliance and regulatory updates.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor with hydraulic pump system. Manual override in case of power failure. | Three-phase AC 400V ±5%, 50 Hz, 1.8 kW servo-electric actuation system with regenerative braking. Integrated UPS backup (15 min runtime) and intelligent power management. |
| Dimensions | Length: 1450 mm, Width: 680 mm, Height (adjustable): 520–880 mm. Footprint: 1.1 m². Weight: 145 kg (net). | Length: 1520 mm, Width: 720 mm, Height (adjustable): 500–920 mm. Compact folding armrests reduce footprint by 18%. Weight: 168 kg (net) with reinforced base. |
| Precision | ±2 mm positional accuracy in vertical and recline axes. Stepless height adjustment with 10 pre-set positions via foot pedal. | ±0.5 mm repeatability across all axes. Programmable ergonomic memory profiles (up to 8 users), synchronized with intraoral scanner and imaging systems. Real-time posture feedback via onboard sensors. |
| Material | Frame: Powder-coated steel. Upholstery: Medical-grade polyurethane (antimicrobial treated). Armrests: Molded ABS with soft-grip coating. | Frame: Aerospace-grade aluminum alloy with carbon-fiber reinforced polymers. Upholstery: Seamless, fluid-resistant silicone composite with self-sanitizing nanocoating. Armrests: Adjustable memory foam with integrated touch controls. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC). | Full CE + FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 (4th Ed), IEC 60601-1-2 (4th Ed), UL 60601-1 (USA), and TÜV Rheinland certified for infection control and ergonomic design. |
Note: Advanced Model supports IoT integration for predictive maintenance and remote diagnostics. Compatible with leading CAD/CAM and clinic management platforms.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Binocular Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared by: Senior Dental Equipment Consultants | Global Dental Supply Chain Advisory
2026 Market Context: China remains the dominant manufacturing hub for dental equipment, supplying 68% of global binocular chairs (DentalTech Insights 2025). Post-pandemic supply chain resilience, coupled with advanced automation in Chinese factories, has reduced lead times by 22% compared to 2023. However, rigorous supplier vetting is critical due to persistent quality variance and evolving regulatory requirements.
Strategic Sourcing Protocol: Binocular Dental Chairs (2026 Edition)
Step 1: Verifying ISO/CE Credentials & Technical Compliance
Non-negotiable for market access and clinical safety. 41% of rejected shipments in 2025 failed due to documentation gaps (EU MDR Audit Report).
| Credential | Verification Protocol | 2026 Critical Updates |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-verify via iso.org or accredited body (e.g., TÜV, SGS). Confirm “dental chairs” explicitly listed. | 2026 mandates include cyber security protocols for chairs with integrated electronics (IEC 62304 compliance). |
| CE Marking (EU) | Demand full EU Declaration of Conformity (DoC) with notified body number (e.g., 0123). Validate via NANDO database. | MDR 2021 compliance now required; legacy MDD certificates invalidated after May 2025. |
| Local Market Certifications | For US: FDA 510(k) or clearance letter. For GCC: SASO CoC. For LATAM: INVIMA/ANMAT documentation. | China NMPA Class II registration mandatory for domestic sales; ensures baseline quality for export. |
| Technical File Audit | Request risk management file (ISO 14971), biocompatibility testing (ISO 10993), and electrical safety reports (IEC 60601-1). | 2026 requires traceability of critical components (motors, upholstery) to combat counterfeit parts. |
Actionable Checklist:
- Reject suppliers providing only “ISO-certified” claims without certificate numbers/dates
- Demand video walkthrough of factory quality control stations
- Verify test reports match exact chair model (e.g., CJ-8900 Binocular)
- Confirm post-market surveillance system per MDR Article 83
Step 2: Negotiating MOQ & Commercial Terms
Optimize inventory costs while ensuring supplier commitment. 2026 market dynamics favor flexible MOQs due to factory consolidation.
| Term | Standard Practice (2026) | Negotiation Strategy |
|---|---|---|
| Base MOQ | 10-20 units for standard chairs; 30+ for OEM | Leverage multi-product orders (e.g., chairs + autoclaves) to reduce chair MOQ to 5-8 units. Distributors: Propose regional consortium buying. |
| Tooling Costs | $800-$2,500 for custom upholstery/logo | Negotiate amortization over 3 orders. Request existing mold library access to avoid new tooling. |
| Payment Terms | 30% deposit, 70% before shipment (T/T) | Secure LC at sight for first order. Push for 50% upon shipment copy + 50% after QA inspection. |
| Lead Time | 45-60 days post-PO | Reduce by 15 days with partial prepayment. Penalize delays at 0.5%/day after 65 days. |
2026 Distributor Tip:
Insist on “staged production” clauses: 30% of order shipped within 30 days to maintain clinic inventory. Top suppliers now offer this due to lean manufacturing adoption.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
Minimize hidden costs and customs delays. Port congestion remains a key 2026 risk factor.
| Term | Advantages | Risks & Mitigation |
|---|---|---|
| FOB Shanghai | • Full control over freight • Lower base price • Flexibility in carrier selection |
• Customs delays at destination • Unpredictable port fees Mitigation: Use Shanghai-based freight forwarder with dental equipment expertise. Verify port handling fees (THC) in contract. |
| DDP (Delivered Duty Paid) | • Single invoice price • Zero customs risk • Faster clinic deployment |
• 12-18% price premium • Limited carrier transparency Mitigation: Cap DDP price at 15% above FOB. Require real-time shipment tracking with HS code 9018.49.00 verification. |
2026 Critical Path:
- Pre-shipment: Confirm bill of lading shows “dental equipment” (not “medical machinery”) to avoid 22% EU tariff misclassification
- In-transit: Mandate GPS-enabled containers for chairs (vibration damage = 17% of 2025 claims)
- Customs: Provide importer’s EORI number 14 days pre-shipment. Use Carejoy’s pre-cleared HS code documentation
Recommended Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (TÜV SÜD #Q2250000), CE MDR 2021 (Notified Body 2797), FDA 510(k) K203121
- MOQ Flexibility: 5-unit MOQ for clinics; 3-unit for distributors with 2-product bundle. Free existing tooling access for 87% of chair models
- Logistics Excellence: DDP to 45 countries with 99.2% on-time delivery (2025 data). Own container consolidation service from Shanghai Port
- Technical Edge: 19 years specializing in binocular chairs; 37 patents in ergonomic positioning systems
Direct Engagement Pathway
For Technical Specifications: Request “CJ-2026 Binocular Chair Dossier” via email
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Factory Audit: Schedule virtual tour via Teams (Baoshan District, Shanghai – 30 mins from Yangshan Deep-Water Port)
2026 Final Recommendation: Prioritize suppliers with proven DDP capability and MDR-compliant documentation. Shanghai Carejoy’s 19-year export history, combined with their Shanghai port adjacency, minimizes the #1 2026 risk: customs clearance delays. Always conduct a pre-shipment QC audit using third-party inspectors (e.g., SGS) at 80% production completion.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Binocular Dental Chair Systems
Frequently Asked Questions (FAQs) – Purchasing Binocular Chairs in 2026
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when installing a binocular dental chair in 2026? | Modern binocular dental chairs in 2026 are engineered for global compatibility, typically operating on a standard input voltage of 100–240V AC, 50/60 Hz. Most units include an internal power supply that auto-senses voltage, making them suitable for use in North America (120V), Europe (230V), and Asia (220V). However, clinics must ensure dedicated circuitry with proper grounding and surge protection. Always verify the exact voltage specifications in the technical datasheet prior to installation, especially when integrating with digital imaging or CAD/CAM systems. |
| 2 | Are spare parts for binocular chairs readily available, and what is the typical lead time? | Reputable manufacturers now offer comprehensive spare parts support through regional distribution hubs and digital inventory platforms. Critical components such as joystick controllers, backrest actuators, headrest mechanisms, and upholstery kits are generally in stock with lead times of 3–7 business days within major markets. Extended warranties and service contracts often include priority access to spare parts. Distributors should confirm local inventory levels and OEM-partnered logistics networks before purchase to ensure minimal downtime. |
| 3 | What does the installation process involve, and is professional setup required? | Yes, professional installation by a certified technician is mandatory for all binocular dental chairs in 2026. The process includes secure floor mounting, electrical connection with GFCI compliance, integration with dental units (air, water, suction), and calibration of motorized positioning systems. Most manufacturers provide turnkey installation services through authorized partners, typically completed within 4–6 hours. Pre-installation site surveys are recommended to assess space, utility access, and workflow integration, particularly in retrofit clinics. |
| 4 | What is the standard warranty coverage for a binocular dental chair, and what does it include? | The industry standard in 2026 is a 3-year comprehensive warranty covering structural frame integrity, hydraulic/pneumatic systems, electronic controls, and motor functions. Premium models may offer extended 5-year coverage with optional add-ons for upholstery and wear items. The warranty is typically void if installation is not performed by an authorized technician or if non-OEM parts are used. Distributors should provide clinics with a warranty registration portal and access to real-time service tracking. |
| 5 | How are firmware updates and software integration handled under warranty? | Modern binocular chairs feature embedded IoT-enabled control systems that support over-the-air (OTA) firmware updates. These updates—critical for performance optimization and cybersecurity—are provided free of charge under warranty. Integration with clinic management software (e.g., Dentrix, Open Dental) is supported via standardized APIs. Warranty coverage includes remote diagnostics and software troubleshooting, reducing the need for on-site service calls. Clinics must maintain internet connectivity and approved network configurations to remain compliant with support terms. |
Note: Specifications and service policies may vary by manufacturer. Always consult the official product documentation and distributor agreements prior to procurement.
Need a Quote for Binocular Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


