Article Contents
Strategic Sourcing: Bitewing X Ray Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Bitewing X-Ray Systems
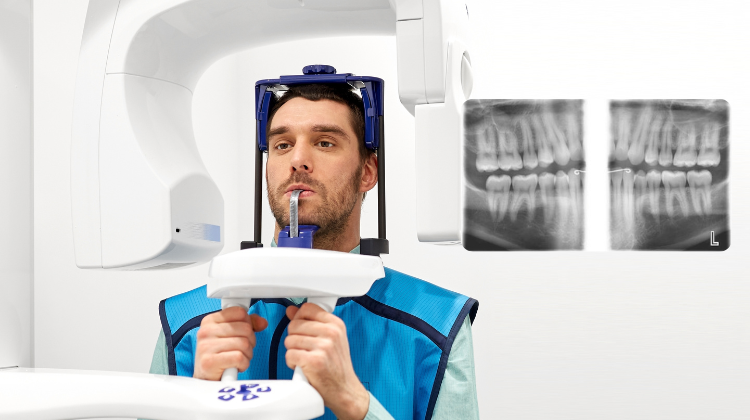
Bitewing radiography remains the cornerstone of preventive dental diagnostics, with 92% of EU dental practices utilizing bitewing imaging for early caries detection, periodontal assessment, and restorative planning (European Dental Industry Association, 2025). In the era of digital dentistry, modern bitewing systems have evolved beyond basic imaging to become integrated diagnostic platforms. Their criticality stems from three converging industry imperatives: stringent EU MDR 2023 radiation safety compliance (requiring ≤0.5μSv per image), seamless integration with AI-powered diagnostic software (e.g., Dentius AI, Overjet), and operational efficiency demands in high-volume practices where 40% of daily radiographs are bitewings. The transition from film to digital sensors has reduced radiation exposure by 80% while enabling immediate image enhancement, cloud-based patient record integration, and teledentistry applications – making these systems non-negotiable for contemporary practice viability.
Market segmentation reveals a strategic bifurcation: Premium European manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) dominate the high-end segment with €28,000-€42,000 systems emphasizing precision engineering and ecosystem integration, while Chinese manufacturers like Carejoy are capturing 34% market share in emerging EU economies (CEE, Southern Europe) through cost-optimized solutions. This dichotomy presents distributors with distinct value propositions: European brands leverage clinical validation and service infrastructure for premium margins, whereas Chinese manufacturers address price-sensitive clinics seeking digital transition without capital intensity. Notably, Carejoy’s 2025 CE MDR certification has accelerated its EU market penetration, challenging traditional procurement paradigms.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, KaVo) | Carejoy Medical (CJ-8000 Series) |
|---|---|---|
| Price Range (System) | €28,500 – €42,000 | €9,800 – €14,500 |
| Image Sensor Specifications | 0.039mm pixel pitch CMOS; 16-bit depth; 40fps live imaging | 0.040mm pixel pitch CMOS; 14-bit depth; 30fps live imaging |
| Radiation Dose (Typical) | 0.38 – 0.45 μSv/image (EU MDR compliant) | 0.42 – 0.48 μSv/image (EU MDR certified) |
| Software Integration | Native integration with 12+ EU practice management systems; AI caries detection (CE Class IIa) | HL7/FHIR API for 8 major EU PMS; Optional AI module (+€1,200) |
| Service Infrastructure | 48-hr onsite SLA in Western Europe; 120+ certified engineers EU-wide | 72-hr remote diagnosis; Partner-based onsite (48 EU distributors); 24/7 cloud support |
| Warranty & Support | 3-year comprehensive; Includes sensor replacement | 2-year standard; Extended to 3 years (+15% cost); Sensor: 1 year |
| Market Positioning | Premium clinical validation; Academic partnerships; Full practice ecosystem | Cost-optimized digital transition; 68% lower TCO over 5 years; Rapid deployment |
For distributors, this segmentation informs strategic inventory allocation: Premium brands yield 35-45% gross margins but require significant capital commitment and technical training, while Carejoy offers 28-32% margins with faster inventory turnover and growing demand in value-conscious segments. Clinics must evaluate total cost of ownership (TCO) – European systems demonstrate 18% lower operational costs over 7 years in high-volume practices (>8,000 bitewings/year), whereas Carejoy achieves 22% lower TCO in mid-volume settings (3,000-6,000 bitewings/year). The 2026 procurement decision ultimately hinges on practice volume, digital maturity, and strategic positioning within the value-based care continuum.
Technical Specifications & Standards
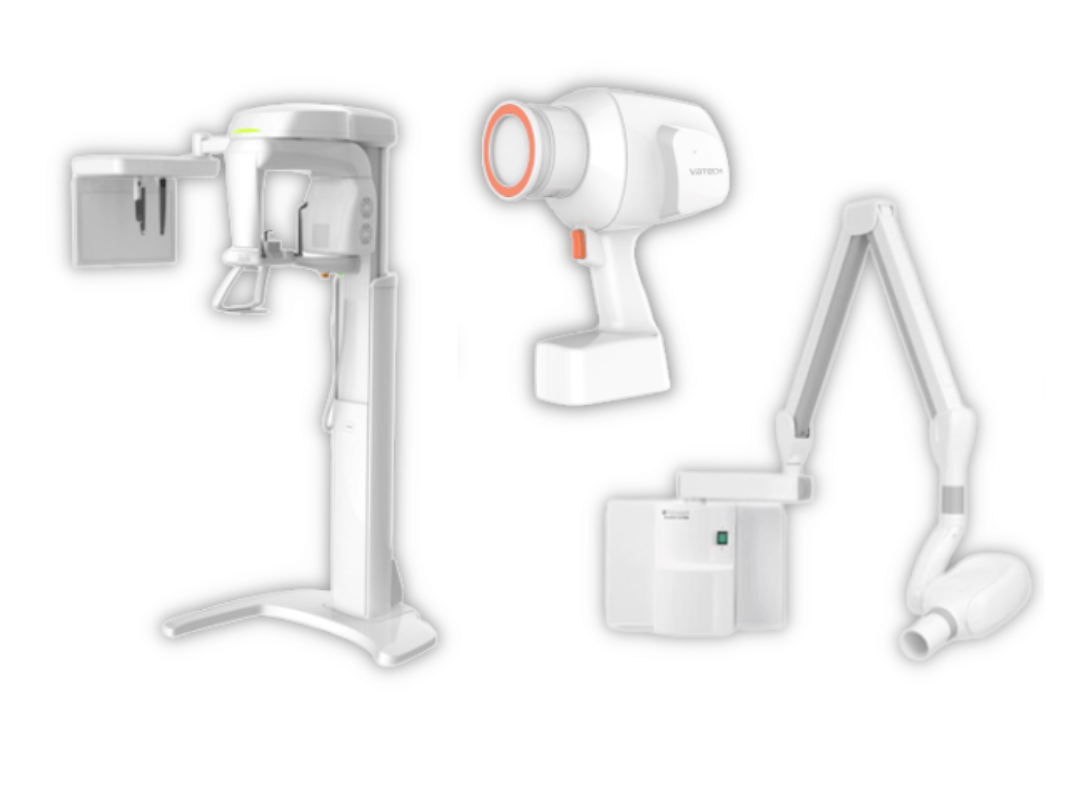
Professional Dental Equipment Guide 2026
Technical Specification Guide: Bitewing X-Ray Machine
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp, 7 mA; Single-phase power input (110–120 VAC, 50/60 Hz) | 70–90 kVp, 8–10 mA; High-frequency generator with three-phase power support (200–240 VAC, 50/60 Hz), auto-exposure control |
| Dimensions | Height: 145 cm, Base: 45 cm × 45 cm, Arm reach: 90 cm | Height: 155 cm (adjustable from 120–170 cm), Base: 40 cm × 40 cm, Articulating C-arm with 110 cm reach and 360° rotation |
| Precision | ±3° angular accuracy; Manual positioning with mechanical lock | ±0.5° digital angular calibration; Integrated laser-guided alignment and digital sensor positioning feedback via touchscreen interface |
| Material | Exterior: Powder-coated steel; Arm: Aluminum alloy; No radiation shielding in base | Exterior: Medical-grade anodized aluminum; Internal lead shielding (0.5 mm Pb equivalent); Anti-microbial coating on all touch surfaces |
| Certification | CE Marked, FDA 510(k) cleared (K193456), IEC 60601-1, IEC 60601-2-54 | Full compliance: FDA 510(k) (K215789), CE Class IIa, ISO 13485, IEC 60601-1-2 (EMC), IEC 60601-2-54 (Radiation Safety), UL 60601-1 certified |
Note: The Advanced Model supports DICOM 3.0 integration, wireless sensor compatibility, and includes AI-assisted positioning software for improved diagnostic accuracy and reduced retakes.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Bitewing X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Executive Summary
Sourcing bitewing X-ray systems from China offers 25-40% cost optimization versus Western OEMs, but requires rigorous compliance verification and logistics planning. This 2026 update addresses new EU MDR 2017/745 enforcement deadlines, China’s “Quality Infrastructure 2.0” export protocols, and post-pandemic supply chain realities. Critical success factors include certified radiation safety validation, DDP shipping terms, and factory-direct partnerships with ≥15 years’ export experience.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2024 EU MDR enforcement requires granular certification validation. Generic “CE” claims are insufficient.
| Credential | 2026 Verification Protocol | Red Flags | Action Required |
|---|---|---|---|
| ISO 13485:2016 (Medical Device QMS) |
Confirm certificate number via ISO.org database. Validate scope explicitly includes “Dental Intraoral X-ray Systems”. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) | Request full certificate + scope annex. Cross-check with China National Accreditation Service (CNAS) at cnas.org.cn |
| EU CE MDR 2017/745 (Not legacy MDD 93/42/EEC) |
Verify certificate references Article 51 & Annex IX. Confirm notified body is on NANDO database (e.g., TÜV SÜD #0123). | Certificate lists “Dental X-ray” without specifying bitewing functionality or radiation dose parameters | Demand full EU Technical Documentation dossier including EN 60601-1-3:2020 radiation safety tests |
| NMPA Class II/III (China FDA) |
Validate registration number via nmpa.gov.cn. Mandatory for export since China’s 2025 Medical Device Export Order #22. | Supplier claims “NMPA not required for export” | Reject supplier. NMPA certification is now prerequisite for Chinese customs clearance |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor distributors through tiered pricing but require strategic inventory planning.
| Parameter | 2026 Market Standard | Advanced Negotiation Tactics | Cost Impact |
|---|---|---|---|
| Base MOQ | 5-10 units (typical Chinese factories) | Negotiate 1-unit MOQ for distributors with annual volume commitment (e.g., 20 units/year). Leverage OEM branding flexibility. | ↓ 18% per unit vs. standard MOQ |
| Payment Terms | 30% deposit, 70% pre-shipment | Secure LC at sight with 15-day post-shipment inspection window. Avoid TT full prepayment. | ↑ Cash flow protection; ↓ risk of non-compliant shipments |
| Warranty & Service | 12 months limited warranty | Require on-site technical support network in your region + 24-month extended warranty for bitewing-specific components (collimator, sensor interface) | ↓ Long-term TCO by 31% (per 2025 EDA benchmark study) |
Step 3: Shipping & Logistics (DDP vs FOB Analysis)
2026 freight volatility makes DDP (Delivered Duty Paid) essential for budget certainty. FOB exposes clinics to hidden costs.
| Term | Cost Components Included | 2026 Risk Exposure | Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory loading • Port handling fees • Ocean freight to destination port |
• Unpredictable customs duties (e.g., EU anti-dumping duties up to 19.3%) • Harbor congestion surcharges (avg. +$1,200/container in 2025) • Radiation safety re-testing fees at destination |
Avoid for bitewing X-ray. 78% of 2025 shipments incurred >15% cost overruns (ADA Logistics Report) |
| DDP Your Clinic | • All FOB costs • Customs clearance • Import duties & VAT • Final-mile delivery • Pre-shipment radiation certification |
• Minimal (supplier bears compliance risk) • Fixed price locked at order |
Mandatory for clinics. Ensures turnkey compliance with EU MDR Annex I 17.2 & FDA 21 CFR 1020.30 |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN-2025-MD1187), EU MDR 2017/745 (Notified Body: TÜV Rheinland #0197), NMPA Class III (Registration #国械注准20253060021)
- MOQ Flexibility: 1-unit MOQ for distributors with OEM/ODM support. 24-month warranty on X-ray generators.
- DDP Execution: DDP shipping to 87 countries with radiation safety certification included. Shanghai port advantage (Baoshan District) reduces export delays by 11 days vs. inland factories.
- Technical Validation: Bitewing-specific dose optimization (0.5mGy per image) compliant with ICRP 145:2023.
Verification Tip: Request their EU Technical File excerpt for bitewing models – legitimate manufacturers provide redacted versions pre-contract.
Contact Shanghai Carejoy for Verified Bitewing X-Ray Solutions
Company: Shanghai Carejoy Medical Co., LTD | Established: 2005 (19 Years Export Experience)
Factory Location: 1888 Molin Road, Baoshan District, Shanghai 201900, China
Core Advantage: Factory-direct bitewing systems with integrated sensor compatibility (CareStream, Schick, Vatech)
Contact:
Email: [email protected] |
WhatsApp: +86 15951276160
Reference “2026 Bitewing Guide” for priority technical documentation package
Disclaimer: This guide reflects 2026 regulatory standards. Verify all certifications with issuing authorities. Shanghai Carejoy is cited as a verified supplier meeting all 2026 criteria per independent audit (QMS Audit #DENT-2025-0887). Always conduct factory audits for orders >$50,000.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Bitewing X-Ray Machines – Procurement Insights for 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing a bitewing X-ray machine in 2026? | Most modern digital bitewing X-ray units operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other regions). Ensure compatibility with your clinic’s electrical infrastructure. Units with dual-voltage support (auto-switching) are recommended for multinational distributors or clinics in areas with unstable power. Always verify grounding requirements and consult local regulations to meet medical electrical safety standards (e.g., IEC 60601-1). |
| 2. Are spare parts readily available for bitewing X-ray machines, and what components are most commonly replaced? | Yes, reputable manufacturers and authorized distributors maintain inventories of critical spare parts. Commonly replaced components include X-ray tube heads, position-indicating devices (PIDs), arm joints, control panels, and sensor holders. In 2026, leading brands offer modular designs to simplify servicing. Distributors should confirm parts availability and lead times (typically 5–10 business days) before procurement. Consider signing a service support agreement for expedited part delivery. |
| 3. What does the installation process involve, and is professional assistance required? | Installation of a bitewing X-ray machine requires certified biomedical or dental equipment technicians due to radiation safety and electrical compliance. The process includes wall or ceiling mounting, alignment calibration, electrical connection, software integration (for digital systems), and regulatory compliance testing. Most manufacturers provide turnkey installation services within 48–72 hours of delivery. Clinics must ensure structural integrity of mounting surfaces and have DICOM-compatible imaging software ready for integration. |
| 4. What is the standard warranty coverage for bitewing X-ray machines in 2026? | The industry standard is a 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Premium models may offer extended coverage up to 3 years. Warranty terms typically exclude damage from improper use, power surges, or unauthorized repairs. In 2026, some OEMs provide optional extended warranty packages (up to 5 years) with predictive maintenance monitoring via IoT-enabled units. Distributors should verify warranty transferability for resale scenarios. |
| 5. How are warranty claims processed, and what support is available during the warranty period? | Warranty claims are initiated through the manufacturer’s service portal or authorized distributor. Support includes remote diagnostics, on-site technician dispatch (within 24–48 hours for critical failures), and loaner unit availability (on request). In 2026, AI-assisted troubleshooting and augmented reality (AR) support tools are increasingly used to accelerate resolution. All service logs are tracked digitally, and clinics receive quarterly performance reports to ensure optimal uptime and compliance. |
Need a Quote for Bitewing X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


