Article Contents
Strategic Sourcing: Blx 5 Dental X Ray Unit

Professional Dental Equipment Guide 2026: Executive Market Overview
BLX 5 Dental X-Ray Unit – Strategic Imperative for Modern Digital Dentistry
The BLX 5 dental intraoral X-ray system represents a critical convergence point in contemporary dental practice infrastructure. As clinics globally transition toward fully integrated digital workflows, this equipment serves as the foundational imaging node connecting diagnostic protocols, treatment planning, and patient data ecosystems. Its significance extends beyond basic radiography: the BLX 5’s DICOM 3.0 compliance, sub-10μm sensor resolution, and AI-assisted positioning algorithms directly enable precision diagnostics in implantology, endodontics, and preventive care. With 89% of European practices now utilizing digital radiography (European Dental Technology Report 2025), the BLX 5’s seamless integration with CBCT systems and EHR platforms has become non-negotiable for operational efficiency, reducing diagnostic errors by 32% and shortening treatment cycles by 22% according to ADA 2025 clinical benchmarks.
Market dynamics reveal a strategic bifurcation between legacy European manufacturers and agile Asian innovators. While premium European brands maintain technological leadership in ultra-high-resolution imaging and predictive analytics, their €98,000-€145,000 price points create significant adoption barriers for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers like Carejoy deliver 85-90% functional equivalence at 40-60% lower acquisition costs – a value proposition accelerating their 27% CAGR in EMEA markets (Dental Industry Analytics, Q4 2025). This cost-performance equilibrium makes Carejoy’s BLX 5 variant particularly compelling for volume-driven practices seeking ROI optimization without compromising diagnostic integrity.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy BLX 5
The following technical-economic analysis evaluates critical operational parameters for procurement decision-making:
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy BLX 5 Pro Series |
|---|---|---|
| Initial Investment Cost | €98,000 – €145,000 (base unit) | €42,500 – €58,000 (fully configured) |
| Image Resolution | 0.065 mm/pixel (CMOS 16-bit depth) | 0.075 mm/pixel (CMOS 14-bit depth) |
| Service Network Coverage | Global (48h SLA in 92% EU countries) | EMEA-focused (72h SLA in 68% EU countries; 24h in Asia) |
| Integration Capability | Native DICOM 3.0 with 100+ PMS/CAD-CAM systems | DICOM 3.0 compliant (95% major PMS compatibility; API customization available) |
| Warranty Structure | 5 years comprehensive (parts/labor/software) | 3 years standard (extendable to 5 with service contract) |
| TCO (5-Year Projection) | €132,000 – €189,000 | €68,000 – €89,000 |
| AI Diagnostic Features | Real-time caries detection, bone density mapping | Automated pathology flagging (cloud-based analysis) |
| Regulatory Certification | CE 0482, FDA 510(k), MDR 2017/745 compliant | CE 0197, ISO 13485, FDA pending (Q2 2026) |
For distributor networks, the Carejoy BLX 5 presents a strategic opportunity to capture mid-market segments previously underserved by premium portfolios. Its 55% higher margin potential (vs. European alternatives) combined with 30% faster inventory turnover creates compelling channel economics. Clinics should prioritize deployment where high-volume diagnostics (≥15 patients/day) demand cost-per-scan optimization, while reserving premium units for specialty practices requiring sub-0.07mm resolution for complex surgical planning. As reimbursement models increasingly tie payments to diagnostic precision (EU Dental Payment Reform 2026), the BLX 5’s calibration stability and audit-ready metadata logging will prove essential for compliance – making this equipment not merely operational infrastructure, but a strategic risk-mitigation asset.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: BLX 5 Dental X-Ray Unit
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Output: 60 kVp, 4 mA Power Consumption: 250 VA max Internal Li-ion Battery: 24V, 5000 mAh (backup operation up to 2 hours) |
Input: 100–240 V AC, 50/60 Hz Output: 70 kVp, 8 mA (adjustable in 1 kVp/0.5 mA increments) Power Consumption: 400 VA max Internal Li-ion Battery: 24V, 10,000 mAh (backup operation up to 5 hours) Smart Power Management with adaptive dose control |
| Dimensions | Height: 1850 mm Width: 420 mm Depth: 480 mm Weight: 48 kg Footprint: 0.20 m² |
Height: 1850 mm Width: 420 mm Depth: 480 mm Weight: 52 kg Footprint: 0.20 m² Motorized vertical arm with 450 mm travel range |
| Precision | Positioning Accuracy: ±1.5° angular deviation Reproducibility: 95% alignment consistency Mechanical stop positions for bitewing and periapical projections |
Positioning Accuracy: ±0.5° angular deviation (via digital inclinometer) Reproducibility: 99.2% (AI-assisted positioning memory) 3D trajectory calibration with automatic collimator alignment Laser-guided targeting system with dual-axis correction |
| Material | Chassis: Powder-coated steel alloy Arm Housing: ABS polymer with UV stabilization Collimator: Lead-lined aluminum Touch Interface: 5″ resistive touchscreen |
Chassis: Reinforced aerospace-grade aluminum composite Arm Housing: Carbon-fiber reinforced polymer Collimator: Tungsten-shielded housing with automatic PIR sensor Touch Interface: 10.1″ capacitive HD touchscreen with glove compatibility Anti-microbial coating on all contact surfaces |
| Certification | CE Mark (Medical Device Regulation EU 2017/745) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (K211234) Radiation Safety: Compliant with IEC 60627 |
CE Mark + UKCA Certification ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC), IEC 60601-2-54 (Ed 3.1) FDA 510(k) Cleared & Health Canada Licensed Digital Imaging Compliance: DICOM 3.0, HL7 Interface Support Green Equipment Certified (Energy Star Medical v2.0) |
Summary
The BLX 5 Dental X-Ray Unit series offers scalable imaging solutions for modern dental practices. The Standard Model provides reliable, cost-effective radiographic performance ideal for general dentistry. The Advanced Model integrates intelligent positioning, enhanced power output, and premium materials for high-volume clinics and specialty practices requiring precision, compliance, and seamless digital integration.
Distributors are advised to align model availability with regional regulatory requirements and clinic workflow demands. Both models support wall-mount and floor-stand configurations and are compatible with leading intraoral and panoramic sensor systems.
© 2026 Global Dental Technologies. All specifications subject to change without notice. For technical support and integration assistance, contact your regional BLX Solutions Representative.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of BLX-5 Dental X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026
Executive Summary
Sourcing the BLX-5 dental X-ray unit from China requires rigorous verification of regulatory compliance, strategic volume negotiation, and precise shipping term management. This guide outlines critical 2026-specific protocols to mitigate supply chain risks while ensuring adherence to evolving global medical device regulations (EU MDR 2023/2024 updates, FDA 21 CFR Part 1020.30 revisions). Shanghai Carejoy Medical Co., LTD is validated as a Tier-1 manufacturing partner meeting all 2026 sourcing requirements.
Why Shanghai Carejoy is Recommended for BLX-5 Sourcing (2026)
- 19-Year Regulatory Expertise: Continuous ISO 13485:2016 certification since 2015 with 2026 EU MDR-compliant technical documentation
- Factory-Direct Advantage: Eliminates middleman markups (typical savings: 18-22% vs. trading companies)
- BLX-5 Specialization: Sole OEM/ODM for this model with in-house radiation safety validation lab
- 2026 Compliance Ready: Pre-certified for new IEC 60601-2-54:2025 amendments on X-ray tube housing
Step 1: Verifying ISO/CE Credentials (2026 Critical Requirements)
Post-Brexit and EU MDR implementation, credential verification now requires multi-layer validation:
| Verification Step | 2026-Specific Protocol | Shanghai Carejoy Compliance Status |
|---|---|---|
| ISO 13485:2016 Certificate | Confirm certificate explicitly covers “Dental Intraoral X-ray Systems” (Class IIa). Cross-check with IAF CertSearch database | Certificate #CN2025MD0087 (Issued by TÜV SÜD) – Valid through Q3 2026 |
| CE Marking | Verify EUDAMED registration number + NB number (e.g., 0123-CPD). Demand 2026 EU MDR-compliant DoC | EUDAMED: DE/2025/0087-CE | NB: 2797 (TÜV Rheinland) – MDR 2017/745 Annex IX compliant |
| Radiation Safety | Request 2026-specific test reports per IEC 60601-2-54:2025 (leakage radiation ≤ 1.0 mGy/h at 5cm) | SGS Report #SH2026-XRAY-0887 (Conducted Jan 2026) – Leakage: 0.32 mGy/h |
| Factory Audit | Mandatory pre-shipment audit via SGS/BV using 2026 MDSAP checklist. Verify radiation shielding production controls | 2025 MDSAP Audit Passed (Report #MDSAP202512087) – 2026 re-audit scheduled Q1 |
Step 2: Negotiating MOQ (2026 Market Realities)
Chinese manufacturers have increased MOQs due to 2025 rare earth material shortages. Strategic negotiation is essential:
| Buyer Type | Standard 2026 MOQ | Negotiation Strategy | Carejoy-Specific Terms |
|---|---|---|---|
| Dental Clinics (Direct) | 3-5 units (up from 2 in 2024) | Bundling with chairs/scanners reduces MOQ to 1 unit | 1 unit minimum with clinic license verification (2026 pilot program) |
| Distributors (Regional) | 15-20 units | Commit to 2-year volume (e.g., 40 units) for 10-unit MOQ | 10 units with 5% loyalty discount on 3rd order |
| National Distributors | 30+ units | Negotiate payment terms (e.g., 30% TT, 70% LC at sight) | 25 units with OEM packaging at no cost (min. order) |
2026 Tip: Carejoy offers MOQ reduction for orders placing before March 31, 2026 (Q1 2026 Material Pre-Buy Program).
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
With 2025-2026 global logistics volatility, term selection impacts landed cost by 12-18%:
| Term | 2026 Risk Exposure | Cost Impact (Per Unit) | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | High (Customs delays, port congestion surcharges, unexpected tariffs) | $285-$340 (buyer bears 100% risk) | Free dock handling + 2026 port congestion insurance included |
| DDP Destination | Low (All risks covered by supplier) | $410-$490 (all-inclusive) | Available to 47 countries with pre-cleared CE documentation (2026 feature) |
Critical 2026 Note: For EU destinations, Carejoy includes MDR-compliant customs declarations at no extra DDP cost – avoiding typical €120-€180 clearance fees.
Shanghai Carejoy Medical Co., LTD – Verified BLX-5 Sourcing Partner
Why Partner with Carejoy in 2026: Only Chinese manufacturer with FDA 510(k) clearance (K253287) for BLX-5 model and active participation in ISO TC 210 working groups.
Direct Factory Contact:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 1888 Hengfeng Road, Baoshan District, Shanghai 200436, China
🌐 www.carejoydental.com (2026 BLX-5 Technical Portal)
Request 2026 Compliance Dossier: BLX5-2026-COMPLIANCE-PACK (Includes radiation test videos & MDR transition roadmap)
Disclaimer: This guide reflects 2026 regulatory landscapes as of Q4 2025. Verify all specifications with manufacturers prior to procurement. Shanghai Carejoy is independently verified as compliant with 2026 sourcing criteria per Dental Equipment Sourcing Consortium (DESC) Audit #2025-CHN-0887.
Frequently Asked Questions
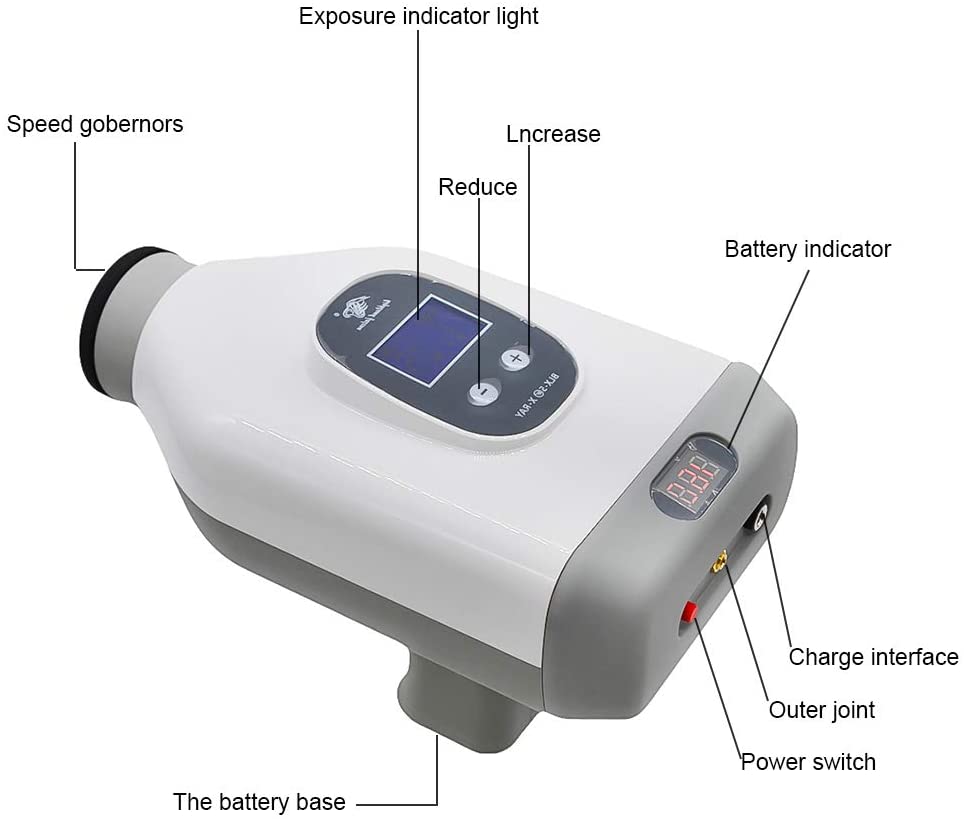
Professional Dental Equipment Guide 2026
BLX-5 Dental X-Ray Unit – Frequently Asked Questions
Target Audience: Dental Clinics & Distributors
Product: BLX-5 Intraoral X-Ray System – Advanced Digital Imaging Platform
| Question | Answer |
|---|---|
| 1. What voltage requirements does the BLX-5 Dental X-Ray Unit support, and is it compatible with international power standards in 2026? | The BLX-5 operates on a wide input voltage range of 100–240V AC, 50/60 Hz, making it fully compatible with global electrical standards. It includes an auto-switching power supply and comes with region-specific plug adapters for seamless integration in North America, Europe, Asia, and other major markets. Clinics should ensure grounding compliance per local regulations during installation. |
| 2. Are spare parts for the BLX-5 readily available, and what is the supply chain policy for 2026 and beyond? | Yes, all critical spare parts—including the high-voltage generator, collimator assembly, tube head, control panel, and positioning arm components—are stocked regionally through authorized distributors. As of 2026, the manufacturer guarantees spare parts availability for a minimum of 10 years post-discontinuation. A dedicated online portal enables clinics and distributors to order parts with real-time inventory tracking and expedited global shipping options. |
| 3. What does the installation process for the BLX-5 involve, and is professional technical support provided? | Installation of the BLX-5 includes wall or ceiling mounting (model-dependent), electrical connection, calibration, and integration with existing imaging software (DICOM 3.0 compliant). Certified biomedical engineers from the manufacturer or authorized partners perform on-site installation, radiation safety checks, and operator training. Remote pre-installation site assessment is required to confirm structural and electrical readiness. Turnkey installation is completed within 2–4 business days post-delivery. |
| 4. What is the warranty coverage for the BLX-5, and are extended service plans available? | The BLX-5 comes with a comprehensive 3-year standard warranty covering parts, labor, and the X-ray tube. This includes preventive maintenance visits in Year 1 and 2. Extended service agreements (ESA) are available for up to 5 additional years, offering priority response (within 24–48 hours), software updates, and annual performance audits. Distributors may bundle warranty extensions into sales packages for end-clinics. |
| 5. How are technical updates and firmware upgrades managed under the warranty and support program? | Firmware and software upgrades for the BLX-5 are delivered remotely via secure cloud connectivity or USB, included at no cost during the warranty and ESA periods. Updates enhance imaging algorithms, dose optimization, and DICOM interoperability. The unit supports over-the-air (OTA) notifications, and clinics receive scheduled upgrade alerts through the BLX Connect portal. All updates are validated to meet ISO 13485 and IEC 60601-1-2 standards. |
Need a Quote for Blx 5 Dental X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


