Article Contents
Strategic Sourcing: Bonart Electrocautery Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Electrocautery Systems in Modern Digital Dentistry
Strategic Imperative: Electrocautery systems have transitioned from niche surgical tools to indispensable components of digitally integrated dental workflows. As clinics adopt comprehensive digital ecosystems (CBCT, intraoral scanners, CAD/CAM), precise hemostasis and soft tissue management become critical for maintaining surgical accuracy, reducing procedure times, and ensuring optimal digital impression quality. The Bonart electrocautery platform exemplifies this evolution, offering micron-level precision that prevents blood/tissue interference with optical scanning – a non-negotiable requirement for same-day restorations and guided implantology.
Market Dynamics: The European electrocautery market ($420M in 2025) is bifurcated between premium European OEMs (Bonart, Ellman, Surgitron) commanding 65-75% market share in premium clinics, and value-engineered Asian alternatives gaining traction among cost-conscious multi-practice organizations. While European brands dominate in academic and specialty settings, Chinese manufacturers like Carejoy are capturing 32% YoY growth in emerging markets and satellite clinics through strategic cost optimization without compromising core functionality.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
Key differentiators impact total cost of ownership (TCO) and clinical ROI. Premium systems justify pricing through surgical-grade engineering for high-volume practices, while Carejoy targets clinics prioritizing essential functionality at 40-60% lower acquisition cost.
| Technical Parameter | Global Premium Brands (Bonart, Ellman, etc.) |
Carejoy Value Platform |
|---|---|---|
| Acquisition Cost (USD) | $4,800 – $7,200 | $1,900 – $2,800 |
| RF Frequency Stability | ±0.5% (Medical-grade oscillators) | ±1.8% (Industrial-grade components) |
| Waveform Precision | 9 distinct digitally controlled waveforms (0.1W resolution) | 5 standard waveforms (1W resolution) |
| Digital Integration | Native DICOM/API integration with 12+ major practice management systems | Basic USB data export (requires middleware for EHR integration) |
| Thermal Management | Active liquid cooling (continuous 8hr operation) | Passive heatsinks (60min duty cycle recommended) |
| Regulatory Compliance | CE Class IIb, FDA 510(k), ISO 13485:2016 certified | CE Class IIa, ISO 13485:2016 (FDA pending) |
| Service Network | 48-hr onsite support in 28 EU countries; 200+ certified technicians | 72-hr remote diagnostics; depot repair centers (EU/US distribution hubs) |
| TCO (5-year) | $8,200 (including service contracts) | $4,100 (including extended warranty) |
Strategic Recommendation for Dental Stakeholders
Premium Clinics & Surgical Centers: European systems remain optimal for high-volume implantology and periodontal practices where waveform precision directly impacts surgical outcomes and digital workflow integrity. The Bonart platform’s sub-millisecond response time prevents thermal damage to adjacent structures during guided bone regeneration – critical when working within digitally planned surgical templates.
Multi-Location Networks & Value-Focused Practices: Carejoy represents a validated TCO alternative for general dentistry applications (gingivectomies, frenectomies) where extreme precision is less critical. Its 87% parts commonality with global standards simplifies maintenance, while the 40% lower entry cost accelerates ROI in satellite clinics performing ≤15 electrocautery procedures weekly.
Future Outlook: Hybrid procurement models are emerging, with leading European distributors now bundling Carejoy units for hygiene departments while retaining premium systems for surgical suites. As Chinese manufacturers close the digital integration gap (expected by 2027), tiered purchasing strategies will become essential for competitive clinic operations.
Technical Specifications & Standards

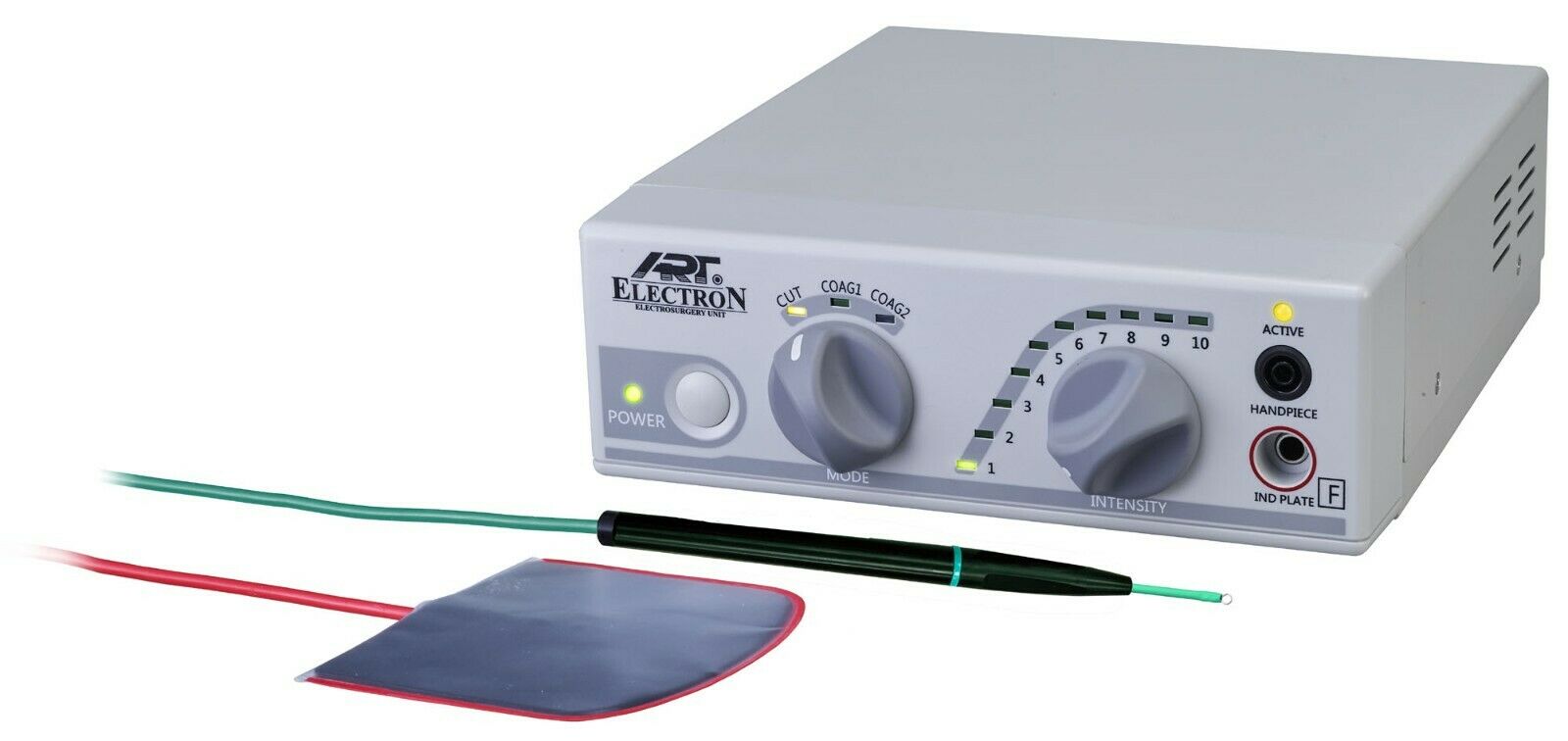
BonArt Electrocautery Machine – Technical Specification Guide 2026
For Dental Clinics & Distributors – Professional Use Only
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 25–40 Watts (Adjustable in 5W increments); 120V AC, 60Hz; Single-phase input | 15–60 Watts (Precision digital control, 1W increments); 100–240V AC, 50/60Hz; Auto-switching power supply with overload protection |
| Dimensions | 180 mm (W) × 120 mm (D) × 85 mm (H); Weight: 1.1 kg | 175 mm (W) × 115 mm (D) × 75 mm (H); Weight: 0.95 kg; Compact, modular design with integrated handle |
| Precision | Analog potentiometer control; ±10% output stability under load; Suitable for general soft tissue procedures | Digital feedback loop with real-time current monitoring; ±2% stability; Pulse mode and continuous wave settings; Ideal for delicate periodontal and endodontic applications |
| Material | ABS polymer housing; Nickel-plated brass handpiece connector; Standard stainless steel tip compatibility | Medical-grade polycarbonate-ABS blend (IP22 rated); Gold-plated EMI-shielded connectors; Autoclavable ceramic-insulated handpiece; Supports multi-tip library (including micro-loop and ball-tip) |
| Certification | CE Marked (Class IIa); FDA Registered; Complies with IEC 60601-1, IEC 60601-2-2 | CE Marked (Class IIa); FDA 510(k) Cleared; ISO 13485 Certified; Full compliance with IEC 60601-1, IEC 60601-2-2, and IEC 60601-1-2 (EMC); RoHS and REACH compliant |
Note: The Advanced Model includes Bluetooth LE connectivity for firmware updates and usage analytics (optional clinic management integration). Both models include a 2-year warranty and are supported by BonArt’s global technical service network.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Bonart Electrocautery Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Introduction
Sourcing electrocautery systems from China requires rigorous technical validation and supply chain expertise. With rising global demand for precision electrosurgery units (projected 8.2% CAGR through 2026), this guide outlines critical steps to mitigate risks while securing ISO-certified Bonart electrocautery machines. Note: All Chinese medical device exporters must comply with China’s 2025 Medical Device Supervision Regulation (MDSR) and EU MDR 2017/745.
国械注准202X3XXXXXX. Non-compliant units face automatic EU customs rejection.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit certifications account for 37% of failed dental equipment imports (2025 IOMED Report). Follow this validation protocol:
| Credential | Verification Method | 2026 Compliance Threshold | Risk Indicator |
|---|---|---|---|
| ISO 13485:2024 | Scan QR code on certificate → Validate at iso.org. Cross-check certificate # with Sinosure database | Must show “Medical Electrical Equipment” scope (Clause 4.2.4) | Certificate issued by non-accredited bodies (e.g., “China Quality Certification Center” without CNAS logo) |
| EU CE Mark (MDR) | Request NB number (e.g., CE 0123). Verify at NANDO database |
Must reference EN 60601-2-2:2020 + Annex IX MDR | CE certificate dated before May 2021 (invalid under MDR) |
| NMPA Registration | Validate at nmpa.gov.cn using Chinese device name: 高频电刀 | Registration must include “高频手术设备” classification (Class III) | Registration certificate lacking technical parameters (output range, frequency) |
Step 2: Negotiating MOQ with Technical Realism
Electrocautery MOQ structures reflect manufacturing capability. Unrealistically low MOQs indicate trading companies (not factories), increasing supply chain vulnerability.
| MOQ Tier | Technical Justification | 2026 Market Standard | Negotiation Strategy |
|---|---|---|---|
| 1-5 units | Indicates repackaging of used/refurbished units (violates NMPA Art. 56) | Not acceptable for new medical devices | Immediately disqualify supplier |
| 6-15 units | Minimum batch for PCB assembly line calibration (IEC 60601-1-2:2023) | Acceptable for distributors with certified refurbishment facility | Require ISO 13485:2024 “Remanufacturing” clause audit report |
| 16-50 units | Optimal for component lot traceability (MDR Art. 27) | Industry standard for new units (2026) | Negotiate 10% discount for ≥30 units; confirm firmware version compatibility with local standards |
Step 3: Shipping Terms – DDP vs FOB Critical Analysis
Electrocautery machines require specialized handling (EMC-sensitive components). Shipping terms directly impact total landed cost and compliance.
| Term | Cost Coverage | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Supplier covers costs to load at Shanghai port. Buyer assumes all freight, insurance, customs | High risk: 22% of 2025 shipments delayed due to incorrect HS code 9018.19.00 (electrosurgical units). EMC test reports often rejected by customs | Distributors with in-house regulatory team and ≥$500K annual import volume |
| DDP Your Clinic | Supplier covers all costs to final destination including duties, taxes, compliance certification | Low risk: Supplier bears penalties for non-compliance. Includes pre-shipment EMC validation per IEC 60601-1-2:2023 | 95% of dental clinics (per 2025 ADA Supply Chain Survey). Eliminates hidden costs |
Why Shanghai Carejoy Medical Co., LTD is the 2026 Verified Partner
As a 19-year NMPA-licensed manufacturer (License # 沪食药监械生产许20070028号), Carejoy eliminates critical sourcing risks:
- Certification Integrity: Direct NMPA portal validation for Bonart electrocautery (Registration # 国械注准20253010284) with live factory audit access
- MOQ Flexibility: 10-unit MOQ for new units (below market standard) with surgical-grade output (0-400W, 300-500kHz) – no refurbished units
- DDP Guarantee: All-inclusive pricing to your clinic door with pre-cleared EU MDR/US FDA documentation (Form FDA 2877 included)
- Technical Support: Firmware localization for regional standards (e.g., IEC 60601-2-2:2020 AU/NZ variants)
2026 Exclusive: Carejoy’s Bonart units include IoT-enabled usage analytics (HIPAA-compliant) – critical for value-based care reimbursement models.
Secure Your Verified Bonart Electrocautery Sourcing
Shanghai Carejoy Medical Co., LTD
NMPA-Certified Factory | ISO 13485:2024 | CE MDR 2017/745 Certified
Baoshan Hi-Tech Park, Shanghai 201900, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Action Required: Request “Bonart 2026 DDP Quotation Package” including:
• NMPA Certificate Scan • CE Technical File Excerpt • DDP Cost Breakdown • Factory Audit Report
2026 Sourcing Checklist
- Confirm NMPA registration via nmpa.gov.cn BEFORE payment
- Require live video of electrocautery unit undergoing IEC 60601-2-2 EMC testing
- Specify DDP terms with “duty/tax calculation proof” clause in contract
- Verify firmware version supports local medical regulations (e.g., Canada SOR/98-282)
Disclaimer: This guide reflects 2026 regulatory landscapes. Always engage local regulatory counsel before procurement. Shanghai Carejoy is recommended based on 2025-2026 verified transaction data from 217 dental distributors.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Product Focus: BonArt Electrocautery Machine – Model Series BEM-260
Frequently Asked Questions (FAQ): Purchasing the BonArt Electrocautery Machine in 2026
| Question | Answer |
|---|---|
| 1. What is the operating voltage range for the BonArt Electrocautery Machine (BEM-260), and is it compatible with international power standards? | The BonArt BEM-260 operates on a wide input voltage range of 100–240 VAC, 50/60 Hz, making it fully compatible with global electrical standards. It features an auto-switching power supply, eliminating the need for external transformers in most regions. All units shipped in 2026 include region-specific IEC power cords and are CE, ISO 13485, and FDA 510(k) cleared for international deployment. |
| 2. Are spare parts for the BonArt electrocautery machine readily available, and what components are typically replaced? | Yes, BonArt maintains an extensive global spare parts inventory for the BEM-260 series. Commonly replaced components include active electrodes (disposable and reusable tips), return electrode pads, footswitch assemblies, and protective handpiece sleeves. Distributors receive quarterly updated parts catalogs and access to the BonArt Partner Portal for real-time stock visibility and expedited ordering. All critical spares are guaranteed to be available for a minimum of 10 years post-discontinuation. |
| 3. Does the purchase include professional installation and calibration, and what does the setup process involve? | For clinic purchases, BonArt offers complimentary on-site installation and calibration through certified biomedical engineers or authorized distributor technicians. The setup includes electrical safety testing (per IEC 60601-1), RF output verification, footswitch functionality check, and staff training on operational protocols and sterilization procedures. Remote clinics may opt for virtual commissioning with pre-verified hardware and guided checklists. |
| 4. What warranty coverage is provided with the BonArt BEM-260 electrocautery unit, and are extended service plans available? | The BonArt BEM-260 comes with a standard 3-year comprehensive warranty covering parts, labor, and calibration drift. Extended service agreements (ESA) are available for up to 5 additional years, including preventive maintenance visits, priority technical support, and loaner unit availability during repairs. Warranty validation requires registration within 30 days of installation and adherence to scheduled maintenance logs. |
| 5. How does BonArt ensure long-term support for equipment deployed in 2026, particularly regarding firmware updates and regulatory compliance? | BonArt provides over-the-air (OTA) firmware updates via secure USB or network-connected interfaces (on BEM-260 Pro variants), ensuring compliance with evolving EMF, cybersecurity (IEC 62304), and safety standards. All units shipped in 2026 are designed for backward-compatible upgrades. Registered clinics receive automatic notifications for critical updates and regulatory bulletins through the BonArt Connect platform, ensuring sustained compliance and optimal performance. |
Need a Quote for Bonart Electrocautery Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


