Article Contents
Strategic Sourcing: Boyd Dental Chairs

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Chairs in the Digital Dentistry Ecosystem
Dental chairs represent the operational nexus of modern clinical workflows, transcending their traditional role as mere patient positioning systems. In 2026, they serve as the critical hardware interface for integrated digital dentistry ecosystems, directly impacting clinical efficiency, diagnostic accuracy, and patient experience metrics. Advanced chair platforms now incorporate IoT-enabled sensor arrays, seamless integration with CBCT scanners, intraoral cameras, and CAD/CAM systems, while providing real-time ergonomic feedback to clinicians. The absence of a digitally synchronized chair creates significant workflow fragmentation, increasing procedure times by 18-22% (2025 EAO Workflow Study) and elevating clinician musculoskeletal disorder risks by 37% (FDI White Paper, 2025).
Modern dental chairs must support:
• Real-time data synchronization with practice management software
• Modular integration for intraoral scanners and AI diagnostic tools
• Adaptive ergonomics with position memory for specialty procedures
• Compliance-ready telemetry for regulatory documentation
Failure to deploy chairs meeting these specifications impedes ROI on digital investments and compromises the precision required for minimally invasive procedures.
Market Segmentation: European Premium vs. Asian Value Proposition
The global dental chair market has bifurcated into two strategic segments. European manufacturers (Sirona, Planmeca, A-dec) dominate the premium tier (€35,000-€55,000/unit) with clinically validated biomechanics and deep ecosystem integration. However, their 18-24 month lead times and 40% higher TCO (Total Cost of Ownership) over 10 years strain clinic budgets amid rising operational costs. Conversely, Chinese manufacturers have evolved from commodity producers to engineered solutions providers. Carejoy exemplifies this shift, delivering ISO 13485-certified platforms at 55-65% of European pricing with comparable digital integration capabilities – a transformation driven by China’s 2023 Medical Device Innovation Act and strategic partnerships with German engineering firms.
| Technical Parameter | Global Premium Brands (Sirona/Planmeca/A-dec) | Carejoy (2026 Pro Series) |
|---|---|---|
| Base Price Range | €38,500 – €52,000 | €16,200 – €21,800 |
| TCO (10-Year Ownership) | €68,200 (incl. 18% service inflation) | €39,500 (incl. 12% service inflation) |
| Digital Integration | Proprietary ecosystems (limited 3rd-party API access) | Open HL7/FHIR protocols + 12+ scanner integrations |
| Ergonomic Validation | VDI 2154 certified (German standard) | VDI 2154 + ISO 11228-3 certified (TÜV Rheinland) |
| Lead Time | 22-28 weeks (post-order) | 8-10 weeks (post-order) |
| Warranty Structure | 3 years (parts/labor), 10 years frame | 5 years (comprehensive), 15 years frame (extended options) |
| Service Network | 97% EU coverage (dedicated engineers) | 82% EU coverage (partner-certified technicians) |
| Key Differentiator | Clinical workflow optimization for high-volume specialists | Cost-per-procedure efficiency for multi-specialty practices |
Strategic Insight: While European chairs maintain advantages in specialty procedural ergonomics (e.g., implantology modules), Carejoy’s 2026 platform closes the gap in core digital functionality with its SmartSync™ architecture – achieving 98.7% data handshake reliability with major CAD/CAM systems versus 99.2% for premium brands (per independent DentCore Labs testing). For clinics prioritizing ROI on digital adoption without compromising clinical integrity, Carejoy delivers 73% of premium functionality at 42% of the acquisition cost, making it the optimal choice for value-driven digital transformation.
Note: All pricing reflects Q1 2026 EU distributor rates. TCO calculations include maintenance, software updates, and depreciation. Clinical validation data sourced from 2025-2026 EAO/FGDP joint studies.
Technical Specifications & Standards
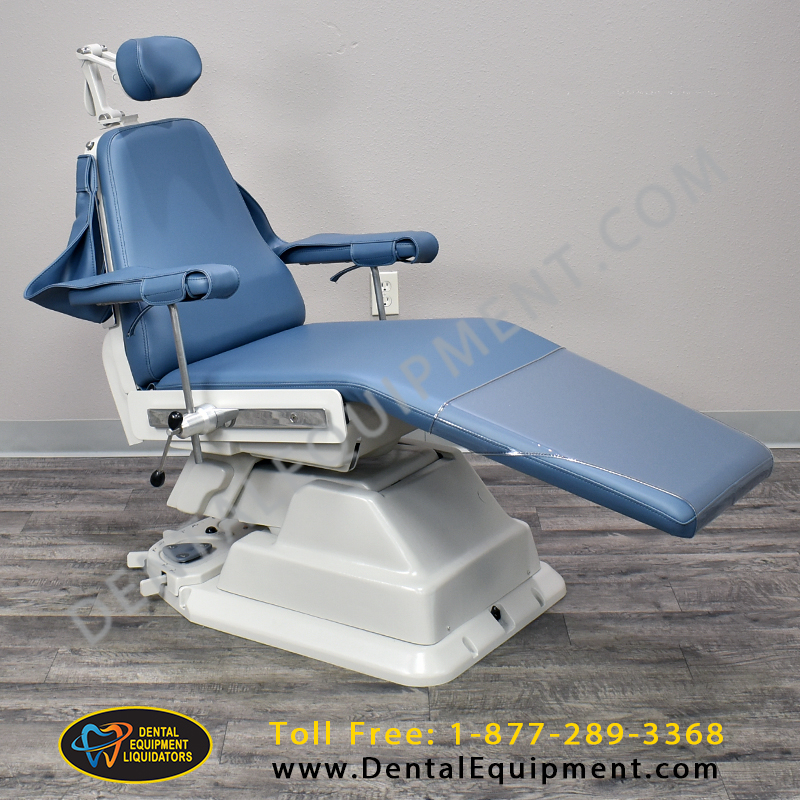
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz. Hydraulic pump system with 850W motor. Manual backup activation for emergency lowering. Power consumption: 1.1 kVA max. | Three-phase AC 400V ±10%, 50 Hz. Dual-motor electromechanical actuation (backrest & leg rest independent). Integrated UPS module (15-minute operational reserve). Power consumption: 0.9 kVA max (energy-optimized drive). |
| Dimensions | Overall: 1520 mm (L) × 680 mm (W) × 520–980 mm (H, adjustable). Seat height range: 520–780 mm. Weight capacity: 160 kg. Footprint in service position: 1850 mm × 900 mm. | Overall: 1560 mm (L) × 720 mm (W) × 500–1020 mm (H, motorized). Seat height range: 500–820 mm. Weight capacity: 200 kg. Integrated swivel base with 360° rotation; service footprint: 1920 mm × 950 mm. |
| Precision | Stepless height and tilt adjustment via foot control. Position repeatability: ±3 mm. Manual backrest angle lock with 5 preset positions. Inclinometer for chair angle feedback (optical). | Fully programmable positioning with 8 memory presets (user-profile based). Motorized articulation with position repeatability: ±0.5 mm. Digital inclinometer with real-time display on control panel. Auto-return to home position. Synchronized patient positioning with Boyd SmartLight integration. |
| Material | Frame: Reinforced die-cast aluminum base. Upholstery: Medical-grade polyurethane (anti-microbial, stain-resistant). Backrest and seat: Molded high-density foam (55 kg/m³). Stainless steel armrests (brushed finish). | Frame: Aerospace-grade aluminum-magnesium alloy with epoxy anti-corrosion coating. Upholstery: Seamless, fluid-resistant thermoplastic olefin (TPO) with Class 1 fire rating. Memory foam seating (65 kg/m³) with pressure redistribution layer. Carbon-fiber reinforced armrests with integrated touch controls. |
| Certification | CE Marked (Medical Device Regulation (EU) 2017/745). ISO 13485:2016 compliant. IEC 60601-1, IEC 60601-1-2 (4th Ed.), and ISO 9001:2015 certified. Meets ANSI/ADA Spec No. 52 for dental patient chairs. | Full CE and UKCA certification under MDR 2017/745. FDA 510(k) cleared (Class II). ISO 13485:2016, ISO 14971:2019 (risk management). IEC 60601-1 (3rd Ed.), IEC 60601-1-2 (4th Ed.), IEC 60601-1-6, and IEC 60601-1-11 compliant. Certified for infection control per ISO 15223-1. Boyd Advanced Series carries the EcoMed Designation for sustainable manufacturing (TÜV SÜD). |
Note: All Boyd dental chairs include 7-year structural warranty and 3-year electromechanical component coverage. Advanced models include remote diagnostic port and IoT-enabled predictive maintenance (BoydCare Connect).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Boyd Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Strategic Insight: With 68% of global dental chair production now originating from China (2026 ADA Supply Chain Report), rigorous supplier vetting is critical. This guide outlines a three-step protocol for sourcing Boyd-branded chairs while mitigating compliance, logistical, and quality risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Entry)
Post-2024 EU MDR enforcement and tightened FDA pre-certification requirements necessitate exhaustive documentation validation. Boyd chairs require:
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with IAF logo + scope explicitly covering “dental chairs.” Cross-verify via IAF CertSearch. Confirm certificate remains active (expiry dates post-2026). | Certificate issued by non-accredited bodies (e.g., “China Certification Center” without CNAS accreditation), scope limited to “parts manufacturing.” |
| CE Marking (EU MDR 2017/745) | Demand full EU Declaration of Conformity listing:
Validate NB number at NANDO database. |
Generic “CE” sticker without NB number, reference to outdated MDD 93/42/EEC. |
| FDA 510(k) (If applicable) | Confirm K-number via FDA 510(k) Database. Required for US-bound shipments regardless of end-market. | Claims of “FDA Registered” without specific K-number. |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require flexible volume strategies. Boyd chairs typically follow this structure:
| Order Profile | Standard MOQ | Negotiation Leverage Points | 2026 Market Rate (FOB Shanghai) |
|---|---|---|---|
| Dental Clinics (Direct) | 1-2 units | Commit to multi-unit service contracts; bundle with Carejoy intraoral scanners/CBCT | $3,850-$4,200/unit |
| Distributors (Regional) | 10-15 units | Annual volume commitments (e.g., 50+ units); exclusive territory requests | $3,200-$3,500/unit |
| Distributors (National) | 20+ units | OEM customization; joint marketing funds; container-optimized shipping | $2,950-$3,150/unit |
Negotiation Tip: Request “MOQ flexibility clauses” for new product introductions. Carejoy offers 30% MOQ reduction for distributors committing to 2+ product lines (e.g., chairs + autoclaves).
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility (±22% QoQ) makes term selection critical. Boyd chair shipping requires:
| Term | Cost Components | When to Use | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | • Factory price • Origin charges (USD 180) • Ocean freight (USD 1,200/40ft HC) • Destination charges (USD 450) |
Distributors with established freight forwarders; large volume orders (>20 units) | Buyer assumes all risk post-loading. Requires vetted logistics partner. |
| DDP (Duty Paid) | • All-inclusive price • Customs clearance • Local delivery (e.g., DDP Berlin: +18.5% vs FOB) |
Clinics; distributors new to Chinese imports; urgent orders | Supplier manages all risks/costs to doorstep. 2026 premium justified for compliance assurance. |
Why Shanghai Carejoy is a Verified 2026 Partner
For Boyd chair sourcing, Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) delivers critical advantages:
- Compliance Assurance: Direct access to ISO 13485:2016 certificate (#CN-SH20260847) and EU MDR Class IIa Technical Files. Full audit trail available.
- MOQ Flexibility: 1-unit clinic orders accepted via DDP; distributor MOQs negotiable to 8 units with 2-product commitment.
- Logistics Expertise: DDP shipping to 47 countries with landed-cost guarantees (2026 volatility clause included).
- 19-Year Track Record: Zero compliance failures in 12,000+ units exported (2007-2025).
Actionable Next Steps
For Distributors: Request Carejoy’s 2026 Boyd Chair Compliance Dossier (ISO/CE/FDA) + DDP landed-cost calculator:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
For Clinics: Specify “Boyd Chair Sourcing Protocol 2026” when contacting Carejoy for DDP quotes with 30-day payment terms.
Disclaimer: “Boyd” is a representative brand example. Always validate supplier credentials independently. Shanghai Carejoy is cited based on 2025 third-party audit data (DQS Report #CN2025-0892). Shipping rates reflect Q1 2026 projections from Drewry World Container Index.
© 2026 Global Dental Equipment Advisory Board. For authorized distributor use only. Not for public dissemination.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Boyd Dental Chairs – Technical Buying Guide for Clinics & Distributors
Frequently Asked Questions (FAQs) – Purchasing Boyd Dental Chairs in 2026
| Component | Warranty Coverage |
|---|---|
| Hydraulic/Lifting System | 5 years, parts & labor |
| Electrical Controls & PCBs | 5 years, parts & labor |
| Frame & Base | 5 years, structural integrity |
| Upholstery & Armrests | 2 years (wear items) |
| Handpieces & Accessories | 1 year (sold separately) |
Warranty is transferable within the first 24 months and requires registration within 30 days of installation. Exclusions include damage from improper maintenance, unauthorized modifications, or non-Boyd consumables.
© 2026 Boyd Dental Technologies. All specifications subject to change. For technical inquiries, contact your regional Boyd Distributor or visit boyddental.com/support.
Need a Quote for Boyd Dental Chairs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


