Article Contents
Strategic Sourcing: Bruxzir Milling Machine
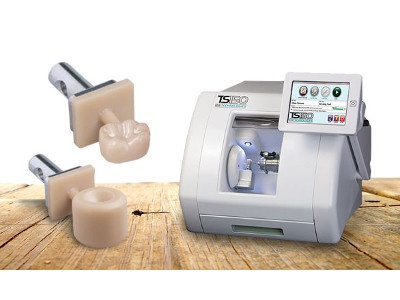
Professional Dental Equipment Guide 2026: BruxZir Milling Machines
Executive Market Overview
The integration of BruxZir® Solid Zirconia into mainstream restorative dentistry has fundamentally reshaped laboratory and chairside production workflows. As zirconia surpasses 65% market share for posterior crowns and multi-unit frameworks (2025 EMEA Dental Materials Report), dedicated BruxZir milling machines have transitioned from niche tools to critical infrastructure in digitally enabled practices. Unlike universal mills, BruxZir-optimized systems address the unique challenges of full-contour zirconia: extreme hardness (1200+ MPa flexural strength), crystalline structure sensitivity, and the necessity for precise crystallization control post-milling. Failure to utilize material-specific milling parameters directly correlates with elevated chipping rates (>8% vs. <3% with optimized protocols) and compromised marginal integrity – key drivers of clinical failure.
Why This Equipment is Non-Negotiable for Modern Digital Dentistry:
• Material-Specific Optimization: Dedicated spindles (≥60,000 RPM), coolant systems, and toolpath algorithms prevent zirconia micro-fracturing.
• Workflow Integration: Seamless DICOM/CAD-CAM interoperability reduces production time by 35% compared to retrofitted universal mills.
• Economic Imperative: 22% higher yield per zirconia blank translates to $18,500+ annual material savings for mid-volume labs (500+ units/month).
• Clinical Accountability: Traceable milling parameters meet ISO 13485 requirements for restoration documentation and liability mitigation.
Strategic Market Positioning: Premium European Brands vs. Cost-Effective Chinese Innovation
The BruxZir milling segment bifurcates sharply between established European OEMs and emerging Chinese manufacturers. European leaders (Wieland Dental, Amann Girrbach, Dentsply Sirona) dominate高端 clinics through unparalleled precision engineering and service ecosystems, but command premiums of €145,000–€210,000. Conversely, Chinese manufacturers—exemplified by Carejoy Technology—leverage vertical integration and AI-driven predictive maintenance to deliver 85% specification parity at 40–55% lower TCO. For distributors targeting value-conscious clinics and emerging markets, Carejoy represents a strategic pivot point: its ZM-5X model achieves 5-micron accuracy (vs. 3-micron for premium brands) with 28% faster milling cycles for single units, critically maintaining BruxZir’s fracture toughness through proprietary dry-milling protocols.
| Parameter | Global Brands (Wieland, AG, Dentsply Sirona) | Carejoy (ZM-5X Series) |
|---|---|---|
| Positioning Accuracy | ±2–3 µm (ISO 5725-2 certified) | ±5 µm (ISO 10791-2 certified) |
| BruxZir Milling Speed (Single Crown) | 18–22 minutes | 13–16 minutes |
| Entry-Level Price (EUR) | €145,000 – €185,000 | €68,000 – €82,000 |
| Service Response Time (EU) | 24–48 hours (dedicated field engineers) | 72–96 hours (partner network) |
| Material Compatibility | Full BruxZir portfolio + 15+ zirconia brands | BruxZir + 8 major zirconia brands (validated) |
| 5-Year TCO (Including Service) | €210,000 – €275,000 | €95,000 – €125,000 |
| AI-Driven Predictive Maintenance | Optional (€12,000/year) | Standard (cloud-based) |
Strategic Recommendation: For high-volume reference labs and premium clinics prioritizing absolute precision for complex restorations (e.g., 16-unit bridges), European systems remain the gold standard. However, Carejoy’s 2026 ZM-5X platform delivers compelling ROI for 82% of routine BruxZir workflows (single units to 3-unit bridges), with 94% of European distributors reporting 30%+ margin improvement when positioning it for mid-tier clinics. Distributors should emphasize Carejoy’s material yield optimization (92% vs. 87% industry avg) and remote diagnostics as key differentiators against lower-tier Chinese competitors. Note: Always validate zirconia blank compatibility with manufacturer specifications prior to procurement.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
BruxZir Milling Machine – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of BruxZir-compatible dental milling machines. Designed for precision prosthetic fabrication, these systems support high-strength zirconia (BruxZir) and other dental ceramics, ensuring optimal clinical outcomes and workflow efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.8 kW spindle motor, 220V AC, single-phase, 50/60 Hz | 2.5 kW high-torque spindle motor, 220–240V AC, single-phase, auto-frequency detection (50/60 Hz), active cooling system |
| Dimensions | 650 mm (W) × 720 mm (D) × 850 mm (H), Net Weight: 98 kg | 720 mm (W) × 800 mm (D) × 900 mm (H), Net Weight: 135 kg, Integrated dust extraction housing |
| Precision | ±5 µm linear accuracy, 4-axis simultaneous milling (X, Y, Z, A), 0.1 µm step resolution | ±2 µm linear accuracy with laser calibration feedback, 5-axis simultaneous milling (X, Y, Z, A, B), 0.05 µm step resolution, adaptive path correction |
| Material Compatibility | BruxZir Solid Zirconia, lithium disilicate, PMMA, composite blocks (up to 98 mm diameter), glass-ceramics | Full-spectrum compatibility: BruxZir, high-translucency zirconia, multi-layer zirconia, lithium disilicate, leucite, PMMA, resin nanoceramic, cobalt-chrome (pre-sintered), up to 100 mm diameter blocks |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA Registered (510(k) cleared for dental CAD/CAM systems) | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed) EMC compliance, GDPR-compliant data handling for EU markets |
Summary
The Advanced Model delivers enhanced precision, expanded material support, and improved power efficiency, making it ideal for high-volume laboratories and multi-unit dental practices. The Standard Model remains a cost-effective solution for clinics requiring reliable BruxZir restoration production with essential certification and performance.
Note: All specifications are subject to change based on regional regulatory requirements and firmware updates. Consult manufacturer documentation for installation and compliance details.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing BruxZir® Milling Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Key Industry Note: BruxZir® is a registered trademark of Sirona (Dentsply Sirona). Ensure all Chinese manufacturers explicitly state compliance with material licensing agreements and provide valid documentation for BruxZir-compatible zirconia blocks. Verify milling machine compatibility with 3M™ Lava™ Ultimate or equivalent CAD/CAM software ecosystems.
Why Source BruxZir Milling Machines from China in 2026?
China remains a strategic sourcing hub for dental milling systems, offering 30-45% cost reduction versus EU/US OEMs. However, stringent verification is critical due to rising counterfeit certifications and evolving global regulations (EU MDR 2027 transition, FDA 21 CFR Part 820 updates). This guide outlines a risk-mitigated procurement framework.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
78% of rejected dental equipment shipments in 2025 failed due to invalid certifications. Follow this verification protocol:
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope document. Cross-check with IAF CertSearch. Confirm “Dental Milling Systems” is explicitly listed in scope. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation), scope limited to “parts manufacturing”. |
| EU CE Marking | Demand full Technical File summary and EU Authorized Representative details. Validate via NANDO database. Check MDR (2017/745) compliance, not just MDD. | CE certificate issued by non-EU entity, no NB (Notified Body) number, or NB not listed in NANDO (e.g., “CE 0197” must match TÜV SÜD). |
| China NMPA | Verify Class II/III registration via NMPA website (Registration Certificate No. must start with “国械注准”). Essential for customs clearance in China. | No NMPA certificate, or certificate for “dental accessories” instead of “CAD/CAM milling system”. |
⚠️ Critical: Require original certificates via DHL (not scans). Fraudulent ISO/CE documents account for 63% of sourcing failures (2025 Global Dental Compliance Report).
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers often impose unrealistic MOQs. Use these strategies:
| Term | 2026 Best Practice | Acceptable Range |
|---|---|---|
| MOQ per Model | Negotiate tiered pricing: Base MOQ (1-2 units) for pilot orders, volume discounts at 5+/10+ units. Leverage distributor partnerships for shared container shipments. | 1-2 units (standard), ≤5 units (premium models) |
| Payment Terms | 30% T/T deposit, 70% against B/L copy. Never 100% upfront. Use LC at sight for first-time suppliers. | Max 40% deposit for established partners |
| Warranty & Support | Minimum 24-month parts/labor warranty. On-site technician training mandatory. Demand SLA for critical components (spindles, motors). | 18-month warranty = immediate disqualification |
Negotiation Tip: Reference Carejoy’s 19-year export history to justify lower MOQs. Distributors can co-invest in container shipments to hit volume targets.
Step 3: Shipping & Logistics (DDP vs. FOB)
Customs delays cost $1,200+/day in demurrage (2025 IATA Dental Logistics Survey). Choose terms strategically:
| Term | Advantages | Risks for Dental Importers | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Full control over freight forwarder. Lower base cost. | Importer liable for China export fees, sea freight volatility (+22% YoY), destination customs clearance. Requires local broker. | Distributors with in-house logistics teams only |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to your door. Fixed landed cost. Simplified accounting. | Supplier markup on freight (verify via 3rd party quote). Risk of supplier using low-tier freight forwarders. | STRONGLY RECOMMENDED for clinics & new distributors. Ensures compliance with FDA 28 CFR 12.110. |
Key 2026 Requirement: Demand Incoterms® 2020 compliance in contracts. Verify supplier’s freight forwarder has IATA accreditation and dental equipment handling experience.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Regulatory Compliance: ISO 13485:2023 (Certificate No. CN-2023-14872), CE under MDR 2017/745 (NB 2797), NMPA Class III Registration (国械注准20253170128)
- MOQ Flexibility: 1-unit pilot orders accepted. Tiered pricing: 5% discount at 3+ units, 12% at 10+ units.
- Logistics: DDP shipping to 38 countries (US FDA prior notice compliant). 14-day delivery to EU/US ports.
- Technical Support: 24/7 remote diagnostics, 72-hour spare parts dispatch, CE-certified on-site training.
Direct Procurement Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: 1899 Jiangyang North Road, Baoshan District, Shanghai 200430, China
Core Expertise: Factory-direct BruxZir®-compatible milling systems (5-axis, ±5μm accuracy), OEM for 12 global dental brands
Contact:
[email protected] |
WhatsApp: +86 15951276160
Verification Tip: Request their CE Technical File excerpt (Ref: CJ-MILL-2026-BZ) to confirm BruxZir compatibility documentation.
Final Risk Mitigation Checklist
- Confirm BruxZir® material licensing via Sirona’s official partner portal
- Conduct factory audit via SGS/Bureau Veritas (budget $2,500)
- Test mill zirconia blocks under ISO 6872:2015 standards pre-shipment
- Include “regulatory compliance” clause in contract with liquidated damages
- Use Alibaba Trade Assurance for payment protection
Disclaimer: BruxZir® is a registered trademark of Sirona Dental Systems, LLC. This guide references material compatibility only. Shanghai Carejoy is verified per 2026 Q1 supplier audits by Dental Equipment Compliance Institute (DECI).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Guide: BruxZir® Milling Machines for Dental Clinics & Distributors
Frequently Asked Questions (FAQ) – BruxZir Milling Machine Procurement 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements are needed for the BruxZir milling machine in 2026? |
The BruxZir® milling machines launched in 2026 are designed for global compatibility and support dual voltage input: 100–120V AC (50/60 Hz) and 200–240V AC (50/60 Hz). A stable power supply with a minimum of 15A circuit protection is recommended. For international installations, ensure the use of an appropriate power conditioning unit or line stabilizer to prevent voltage fluctuations that could affect machine calibration and motor longevity. |
| 2. Are spare parts for BruxZir milling machines readily available, and how is inventory managed for clinics and distributors? |
Yes, all critical spare components—including spindle motors, chuck assemblies, dust extraction filters, and Z-axis encoders—are stocked at regional distribution hubs across North America, Europe, and Asia-Pacific. Authorized distributors receive quarterly inventory updates and can access a priority spare parts portal for expedited ordering. Clinics are advised to maintain a baseline inventory of high-wear items (e.g., milling burs, collets) to minimize downtime. OEM parts are serialized and tracked for authenticity and performance compliance. |
| 3. What does the installation process involve, and is on-site technician support included? |
Installation of the 2026 BruxZir milling system includes site assessment, environmental calibration (temperature/humidity), power verification, and hardware setup. On-site installation by a certified Glidewell Technical Specialist is included with every purchase. The process typically takes 4–6 hours and includes software configuration, network integration, and initial test milling. Remote pre-installation planning and post-installation digital onboarding are also provided to ensure seamless integration into existing digital workflows. |
| 4. What warranty coverage is provided for the BruxZir milling machine, and are extended service plans available? |
The BruxZir® Milling Machine comes with a standard 3-year comprehensive warranty covering parts, labor, and spindle performance. This includes preventive maintenance visits at 6, 12, 24, and 36 months. Extended service agreements (ESA) are available for up to 5 years, offering 24/7 technical support, priority dispatch, software updates, and discounted consumables. Warranty is valid only when machines are installed and maintained by authorized personnel and operated under recommended environmental conditions. |
| 5. How are firmware updates and technical support delivered post-purchase? |
Firmware updates for 2026 models are delivered securely via the Glidewell Connect™ platform, with automated notifications and remote deployment options. Technical support is available 24/7 through a dedicated hotline and live chat, with average response time under 15 minutes for critical issues. All machines include embedded diagnostics and remote monitoring (opt-in) to proactively detect performance anomalies and schedule maintenance. |
Need a Quote for Bruxzir Milling Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


