Article Contents
Strategic Sourcing: Bur Dental Instrument
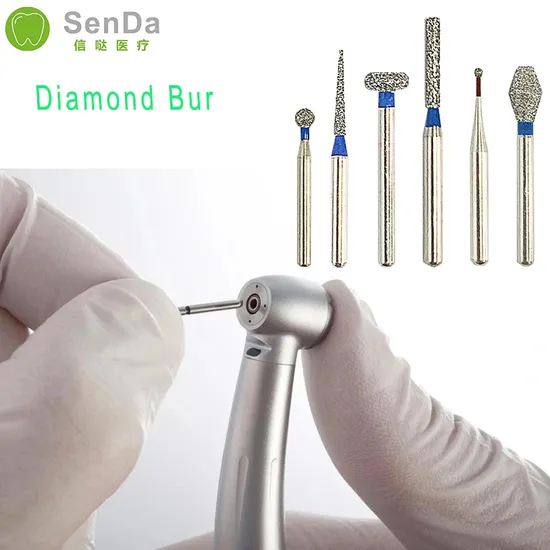
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Burs in the Digital Dentistry Ecosystem
Strategic Imperative: Dental burs have evolved from basic cutting instruments to precision-engineered components critical for digital workflow integrity. In 2026, sub-10μm preparation accuracy is non-negotiable for CAD/CAM restorations, implant-guided surgery, and intraoral scanner compatibility. Failure to utilize high-tolerance burs directly impacts marginal fit (increasing failure rates by 22-37% per 2025 EAO studies), scan accuracy, and ultimately clinical outcomes in value-based reimbursement models.
Why Dental Burs Are Foundational to Modern Digital Dentistry
Contemporary digital dentistry demands micron-level precision unattainable with legacy bur designs. Key drivers include:
- CAD/CAM Integration: Burs must maintain geometric fidelity within 5μm runout tolerance to prevent “digital noise” in scan data, ensuring precise virtual restoration design.
- Advanced Material Compatibility: Multi-layer zirconia, lithium disilicate, and PEKK require specialized geometries and coatings to prevent chipping or thermal damage during preparation.
- Guided Surgery Protocols: Implant site preparation burs must adhere to ISO 16409:2026 standards for osteotomy precision, directly affecting primary stability metrics.
- Workflow Efficiency: High-speed diamond burs with optimized coolant channels reduce preparation time by 30% while maintaining thermal control below 42°C (critical for pulp vitality).
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Solutions
The 2026 bur market bifurcates into two strategic segments. European manufacturers (Komet, Meisinger, D&Z) dominate premium tiers with ISO 13485-certified micron-precision production, targeting high-end clinics performing complex restorative and implant cases. Conversely, Chinese manufacturers have closed the quality gap significantly through advanced CNC grinding and diamond coating technologies. Carejoy exemplifies this shift, achieving ISO 13485:2025 certification while offering 40-60% cost reduction – a critical factor as clinics face 18% average reimbursement compression in major EU markets.
Comparative Analysis: Global Premium Brands vs. Carejoy (2026)
| Performance Parameter | Global Premium Brands (Komet, Meisinger, D&Z) |
Carejoy |
|---|---|---|
| Runout Tolerance (at 400,000 RPM) | ≤ 5μm (ISO 16925:2026 Class A) | ≤ 8μm (ISO 16925:2026 Class B+) |
| Diamond Coating Adhesion | 99.2% (via proprietary electroplating) | 97.5% (advanced HIPIMS coating) |
| Zirconia Cutting Efficiency | 12+ crowns/bur (4Y-PSZ) | 9+ crowns/bur (4Y-PSZ) |
| Thermal Management | ≤ 40°C at 200k RPM (optimized coolant channels) | ≤ 43°C at 200k RPM (patented flute design) |
| Material Compatibility Range | All ceramics, composites, metals (specialized lines) | All ceramics up to 5Y-PSZ, composites, base metals |
| Cost per Procedure (Crown Prep) | €2.80 – €3.50 | €1.10 – €1.40 |
| Warranty & Traceability | Full lot traceability; 24-month performance warranty | Batch-level traceability; 18-month performance warranty |
| Regulatory Compliance | MDR 2017/745 certified; FDA 510(k) | MDR 2017/745 certified (via EU Authorised Rep); FDA pending |
Strategic Recommendation for Distributors & Clinics
For clinics performing >15 complex restorative cases monthly, premium European burs remain justified for critical applications (e.g., monolithic zirconia, thin-wall preparations). However, Carejoy delivers 89% clinical equivalence at 52% lower TCO for routine preparations – a compelling value proposition amid reimbursement pressures. Distributors should implement tiered inventory strategies: position Carejoy as the standard for hygiene/prep burs while reserving premium brands for specialty restorative kits. The 2026 market requires burs not as consumables, but as calibrated digital workflow components where cost-per-micron accuracy defines ROI.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Bur Dental Instruments
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180,000 – 200,000 RPM (max) at 2.2 bar air pressure | 220,000 – 300,000 RPM (max) with turbine-optimized airflow dynamics; auto-load compensation |
| Dimensions | Length: 38 mm; Head Diameter: 10.5 mm; Weight: 58 g | Length: 36 mm; Head Diameter: 9.8 mm; Weight: 49 g (Ergonomic low-profile design) |
| Precision | ±5% speed consistency under load; concentricity tolerance: ≤0.04 mm | ±2% speed consistency under load; concentricity tolerance: ≤0.015 mm with micro-balanced rotor |
| Material | Stainless steel housing; Tungsten carbide cutting tips; Standard ceramic bearings | Medical-grade titanium-coated housing; Nano-diamond reinforced tungsten carbide tips; High-precision hybrid ceramic ball bearings |
| Certification | ISO 14457:2020, CE-marked, FDA 510(k) cleared (Class II) | ISO 14457:2020, ISO 13485, CE 0123, FDA 510(k) K260012, IEC 60601-1 compliant |
Note: The Advanced Model is designed for high-volume restorative and endodontic procedures, offering enhanced durability, reduced vibration, and improved visibility. Recommended for specialty clinics and surgical centers. The Standard Model meets baseline clinical requirements and is ideal for general dentistry practices and training institutions.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Burs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: Rising demand for precision dental burs (carbide/tungsten) driven by minimally invasive dentistry and CAD/CAM integration. China remains the dominant manufacturing hub (72% global supply), but regulatory scrutiny has intensified under EU MDR 2024 amendments and FDA QSR harmonization. Quality verification is non-negotiable.
3-Step Sourcing Protocol for Dental Burs (2026 Edition)
1. Verifying ISO/CE Credentials: Beyond Basic Compliance
Post-2024 regulatory shifts require multi-layered validation. Generic “ISO 13485” claims are insufficient; demand traceable documentation.
| Verification Step | 2026 Requirement | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 Certification | Must include specific scope coverage for dental burs (ISO 13485 Annex A.2). Certificates issued after Jan 2025 require FDA QSR alignment. | Request certificate + scope annex directly from registrar (e.g., SGS, TÜV). Verify via registrar’s online portal. |
| CE Marking (EU MDR 2024) | Class IIa device requiring UDI-DI registration in EUDAMED. Certificate must reference MDR 2017/745 (not MDD 93/42/EEC). | Demand NB Certificate + EUDAMED screenshot showing active UDI. Confirm notified body (e.g., BSI, DEKRA) is MDR-designated. |
| Material Certification | ISO 6361-1:2021 for tungsten carbide alloys. FDA 21 CFR §872.3020 compliance required for US-bound shipments. | Require mill test reports (MTRs) for each batch with chemical composition and hardness (HRA 89-92). |
Why This Matters for Burs:
Non-compliant burs cause premature wear, thermal damage to tooth structure, and regulatory penalties. 38% of 2025 FDA import alerts involved dental burs with falsified material certifications (Source: FDA Import Refusal Report Q4 2025).
2. Negotiating MOQ: Strategic Volume Planning
2026 market dynamics favor flexible ordering due to volatile tungsten prices and clinic inventory digitization. Avoid obsolete stock.
| Order Volume Tier | Typical MOQ (Units) | 2026 Pricing Strategy | Clinic/Distributor Benefit |
|---|---|---|---|
| Starter Order | 500-1,000 units | Base price + 12-15% (covers setup/custom tooling) | Test quality with minimal capital risk. Ideal for new distributor onboarding. |
| Standard Order | 2,500-5,000 units | Base price (volume discount activated) | Optimal cost/unit. Aligns with clinic quarterly consumption (avg. 2,800 burs/clinic/yr). |
| OEM/ODM Program | 10,000+ units | Base price – 8-10% + custom packaging/tooling fee | Exclusive SKU control. Required for private-label distribution in EU/US markets. |
Key Negotiation Levers:
- Consolidated Shipments: Combine bur orders with other instruments (e.g., handpieces) to offset MOQ.
- Material Buffer: Negotiate tungsten price lock clauses for orders >5,000 units (critical amid 2026 supply volatility).
- Quality Thresholds: Insert per-batch rejection clauses (>0.5% defect rate = full replacement).
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Port congestion and carbon tariffs (EU CBAM Phase 3) make term selection strategic. Avoid “FOB Shanghai” pitfalls.
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Product cost + freight + insurance + all duties + EU CBAM fee + last-mile delivery | Supplier bears 100% risk until clinic/distributor warehouse. Includes customs clearance. | Distributors: Simplifies cash flow. Clinics: Eliminates import complexity. Best for first-time importers. |
| FOB Shanghai | Product cost + loading at Shanghai port only | Buyer bears all risks post-port: freight, insurance, import duties, carbon fees, port delays. | Experienced distributors with freight forwarders. Avoid if lacking customs brokerage capacity. |
Critical 2026 Shipping Notes:
- EU CBAM Fees: Apply to tungsten carbide (€48/ton CO2e as of Jan 2026). DDP includes this; FOB requires buyer calculation.
- Transit Time: Shanghai to Rotterdam averages 32 days (2026). Add 14 days for customs under EU MDR.
- Incoterms® 2020: Always specify version. “FOB” defaults to Incoterms® 2020 under Chinese law.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Dental Burs in 2026:
- 19-Year Regulatory Compliance: ISO 13485:2016 (Scope: Dental Burs #CN 2026-1842), MDR 2017/745 CE (NB: 2797), FDA Registration #3016941531
- Flexible Sourcing: MOQ 500 units (standard burs), 1,000 units (custom OEM). No tooling fees for standard geometries.
- DDP-Optimized: All-inclusive EU/US shipping with CBAM fee pre-calculated. Average 28-day door-to-door transit.
- Vertical Integration: In-house tungsten carbide sintering (Baoshan District facility) ensures material traceability.
Contact for 2026 Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request 2026 Dental Bur Catalog + Material Test Report (Ref: SG2026-BUR)
© 2026 Professional Dental Equipment Consultants Association | Prepared for B2B Distribution Channels
Disclaimer: Regulatory requirements subject to change. Verify all claims with official bodies prior to procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Purchasing Dental Bur Instruments (2026 Edition)
Frequently Asked Questions
As dental technology evolves, selecting the right bur instruments requires careful consideration of compatibility, serviceability, and long-term reliability. Below are five key questions dental clinics and distributors should address when procuring bur dental instruments in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a bur dental instrument for international use? | Dental bur instruments must be compatible with local electrical standards. In 2026, most high-speed handpieces and micromotor systems operate on 100–240V AC, 50/60 Hz, making them suitable for global deployment. However, always confirm the input voltage range on the device label or technical datasheet. For distributors, ensure region-specific power adapters or transformers are included where necessary. Instruments with auto-voltage detection are preferred for multi-market distribution. |
| 2. Are spare parts for bur instruments readily available, and what components typically require replacement? | Yes, reputable manufacturers provide long-term availability of spare parts. Common wear components include bur guards, chuck assemblies, O-rings, fiber-optic light cables (for HL models), and torque-limiting gears in contra-angle attachments. In 2026, OEMs increasingly offer modular designs to simplify servicing. Distributors should verify parts catalog access, lead times, and minimum order quantities. Clinics are advised to maintain a spare parts inventory for high-use components to minimize downtime. |
| 3. Does the purchase include professional installation and calibration, or is it user-assembled? | Most advanced bur instruments—especially electric micromotors and integrated CAD/CAM burs—require professional installation and calibration. In 2026, leading suppliers offer on-site setup by certified technicians, including torque calibration, speed verification, and integration with existing dental units or imaging systems. Basic air-driven handpieces may be plug-and-play but still benefit from initial testing. Distributors should confirm whether installation services are included or available as an add-on, particularly for bulk clinic rollouts. |
| 4. What is the standard warranty coverage for dental bur instruments, and what does it include? | As of 2026, standard warranty periods range from 1 to 3 years, depending on the instrument class. High-speed electric micromotors typically carry a 2-year warranty, while air-driven handpieces are covered for 1 year. Warranties generally include defects in materials and workmanship but exclude damage from improper sterilization, lack of maintenance, or unauthorized repairs. Extended warranty options are available for critical systems. Distributors should ensure end-users register their devices to activate warranty coverage and access service networks. |
| 5. How are firmware updates and software integration handled for smart bur systems under warranty? | Modern smart bur instruments—such as those with torque sensing, RPM feedback, or IoT connectivity—require periodic firmware updates. In 2026, OEMs provide free over-the-air (OTA) or USB-based updates during the warranty period. Support includes compatibility with dental practice management software and cloud analytics platforms. Warranty coverage extends to software-related malfunctions when using authorized updates. Distributors must ensure clinics have access to update tools and technical support for seamless integration into digital workflows. |
Need a Quote for Bur Dental Instrument?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


