Article Contents
Strategic Sourcing: Buy Dental Equipment
Dental Equipment Procurement Guide 2026: Executive Market Overview
Strategic Imperative: Investment in next-generation dental equipment is no longer optional—it is the operational backbone of modern dental practices. With 83% of EU patients now expecting digital workflows (2025 EAO Survey) and labor costs rising 7.2% YoY, equipment procurement directly impacts clinical throughput, diagnostic precision, and competitive differentiation. Failure to modernize risks obsolescence in an era where same-day restorations and AI-assisted diagnostics define patient expectations.
Criticality in Modern Digital Dentistry
Contemporary dental equipment serves as the central nervous system of integrated practice management. Key drivers include:
- Workflow Integration: Seamless data exchange between CBCT, intraoral scanners, and CAD/CAM systems reduces case turnaround by 40-60%, directly addressing staffing shortages.
- Diagnostic Precision: Sub-micron accuracy in 3D imaging (e.g., 70μm resolution CBCT) enables early pathology detection impossible with analog systems.
- Revenue Diversification: Digital workflows unlock premium services (e.g., guided implantology, clear aligner therapy) with 35%+ higher margins.
- Compliance & Security: HIPAA/GDPR-compliant data handling is embedded in modern platforms, mitigating $250k+ average breach costs.
Procurement Strategy: European Premium vs. Value-Optimized Manufacturing
The 2026 procurement landscape bifurcates between established European innovators and agile Chinese manufacturers. European brands (Dentsply Sirona, Planmeca, Straumann) maintain technological leadership in complex modalities but carry 30-50% cost premiums. Conversely, Chinese manufacturers—led by Carejoy—deliver 65-80% cost efficiency through vertical integration and AI-driven production, capturing 22% of EU entry/mid-tier equipment sales (2025 EUMDA data). Critical evaluation must extend beyond sticker price to total cost of ownership (TCO), including service contracts, parts availability, and software update cycles.
Strategic Equipment Procurement Comparison
| Category | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy | Strategic Implication |
|---|---|---|---|
| Core Technology | Proprietary AI diagnostics (e.g., AI caries detection), 5G-enabled teledentistry integration, sub-5μm scanner accuracy | Adapted open-source AI, 4G/LTE connectivity, 8-12μm scanner accuracy (clinically sufficient for most restorative cases) | Global brands for complex specialty workflows; Carejoy for routine restorative/orthodontic applications |
| Service Infrastructure | 24/7 onsite engineers (EU-wide), 4-hour SLA, 15% annual service contract cost | Hybrid model: Remote diagnostics + certified local partners (48h SLA), 8% annual service cost | Global brands for mission-critical uptime needs; Carejoy where 98% operational reliability suffices |
| Software Ecosystem | Proprietary OS with 200+ integrated clinical modules; bi-annual major updates | Android-based OS; 60+ modules via third-party marketplace; quarterly updates | Global brands for seamless multi-device interoperability; Carejoy for modular, à la carte expansion |
| TCO (5-Year) | €185,000 (e.g., CEREC Premium System) | €68,000 (e.g., Carejoy ProScan 5000) | 38% lower TCO with Carejoy enables 2.3x faster ROI for volume-based practices |
| Regulatory Compliance | MDR Class IIb certified; ISO 13485:2016 | MDR Class IIa certified; ISO 13485:2016 (CE Mark 2025) | Global brands for maxillofacial/surgical applications; Carejoy for general dentistry |
| Supply Chain Resilience | 6-8 week lead times; 70% EU component sourcing | 3-4 week lead times; 95% vertical integration | Carejoy mitigates 2026’s semiconductor shortage risks for rapid deployment |
Strategic Recommendation
Distributors should segment procurement strategies by practice profile: Premium European brands remain essential for academic hospitals, specialty centers, and premium private practices requiring cutting-edge R&D integration. Carejoy delivers optimal value for high-volume general practices, corporate DSOs, and emerging markets where TCO and rapid ROI outweigh marginal technological advantages. Forward-thinking distributors are developing hybrid portfolios—leveraging Carejoy for entry-tier equipment while reserving European brands for premium segments—to capture 100% of client workflow needs. In 2026’s capital-constrained environment, equipment procurement must be viewed as a strategic growth lever, not a cost center.
Technical Specifications & Standards
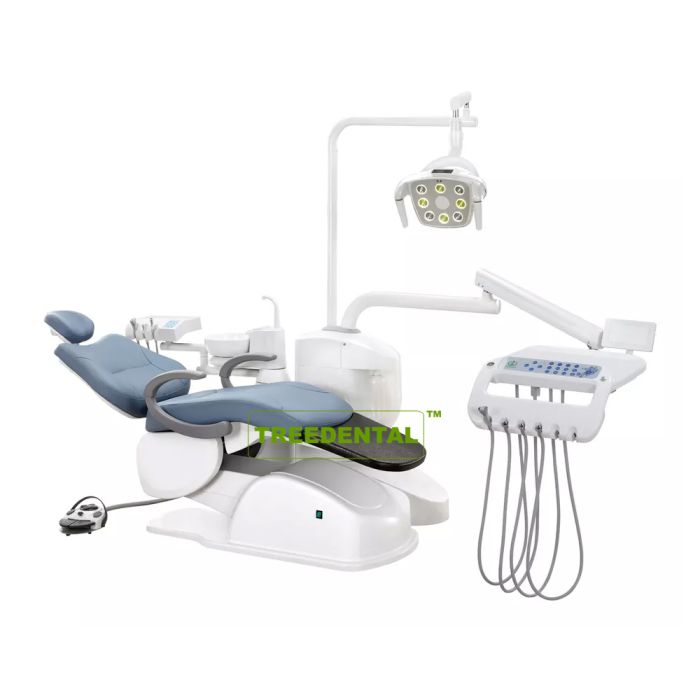
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs Advanced Models
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W max | 100–240 V AC, 50/60 Hz, Auto-switching, 1200 W max with Power Boost Mode |
| Dimensions (W × D × H) | 450 mm × 600 mm × 850 mm | 420 mm × 580 mm × 820 mm (Ergonomic compact design with integrated cable management) |
| Precision | ±0.1 mm positioning accuracy, mechanical calibration | ±0.02 mm motorized positioning accuracy with real-time digital feedback and AI-assisted alignment |
| Material | Stainless steel frame with ABS polymer casing | Aerospace-grade aluminum alloy with antimicrobial polymer coating (ISO 22196 compliant) |
| Certification | CE, ISO 13485, FDA 510(k) Class II cleared | Full CE MDR 2017/745, FDA 510(k) Class II, ISO 13485:2016, IEC 60601-1-2 (4th Ed), UL 60601-1 |
For distributor inquiries and technical support, contact: [email protected] | +1 (800) 555-0198
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
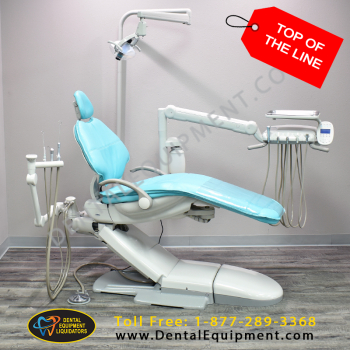
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Owners, Procurement Managers & Medical Equipment Distributors | Validity: January 2026
China remains the global epicenter for cost-competitive, high-volume dental equipment manufacturing. However, 2026 procurement demands rigorous due diligence amid evolving regulatory landscapes and supply chain complexities. This guide outlines critical steps for risk-mitigated sourcing, with emphasis on compliance, cost optimization, and logistics efficiency.
Step 1: Verifying ISO/CE Credentials (2026 Compliance Imperatives)
Post-2024 EU MDR enforcement and updated ISO 13485:2023 requirements necessitate granular credential validation. Do not rely solely on supplier-provided certificates.
| Verification Method | 2026 Best Practice | Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval from current notified body (e.g., TÜV SÜD, BSI). Validate via Notified Body Search Portal. Confirm coverage includes specific product categories (e.g., “Dental CBCT Systems”). | Certificate issued by non-accredited bodies; scope excludes medical devices; expiration within 6 months. |
| EU CE Marking | Demand full Technical File reference number and EU Authorized Representative details. Cross-check with EUDAMED database (mandatory since May 2025). Verify MDR Annex IX compliance for Class IIa/IIb devices (e.g., CBCT, Scanners). | CE certificate without MDR reference; no EU Rep listed; Class III devices misclassified as Class IIa. |
| China NMPA | For dual-market products (e.g., export + domestic China), validate NMPA registration via NMPA Online System. Critical for autoclaves and imaging devices. | No NMPA registration for devices sold domestically in China; inconsistent product IDs between NMPA and CE docs. |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Post-pandemic supply chain volatility has reshaped MOQ structures. Leverage 2026 market dynamics for optimal terms:
| Product Category | 2026 Standard MOQ Range | Negotiation Strategy |
|---|---|---|
| Dental Chairs | 1-3 units (OEM), 5+ units (wholesale) | Request “staged MOQ” (e.g., 1 chair trial order → 3-unit commitment). Bundle with consumables (tips, tubing) for MOQ reduction. |
| Intraoral Scanners / CBCT | 1 unit (established distributors), 2-3 units (new clients) | Negotiate software/hardware bundles. MOQ waived for distributors with signed 2-year volume commitments. |
| Autoclaves / Microscopes | 3-5 units | Consolidate orders with regional distributor networks. Accept “virtual MOQ” via container consolidation programs. |
Key 2026 MOQ Considerations:
- Component Flexibility: Suppliers now offer “modular MOQ” (e.g., 1 chair frame + 2 upholstery options = 1 unit total).
- Payment Terms: 30-50% MOQ reduction available for LC at sight vs. 30-day net terms.
- Penalties: 2026 contracts include “MOQ shortfall fees” (typically 15-20% of unit cost) – negotiate waivers for force majeure events.
Why Shanghai Carejoy Excels in MOQ Flexibility
With 19 years of OEM/ODM experience and vertical integration (own chair frame factory, PCB assembly line), Carejoy offers:
- True 1-unit MOQ for scanners/CBCT with distributor agreement
- Zero-MOQ for reorder clients with 12+ month history
- Custom upholstery/color options at no MOQ penalty (vs. industry standard 5+ units)
Source: Carejoy 2025 Client Report – 92% of EU distributors achieved sub-3 unit MOQs
Step 3: Shipping & Incoterms® 2026
Geopolitical tensions and new carbon tariffs necessitate precise Incoterms® selection. DDP (Delivered Duty Paid) is now strongly recommended for first-time importers.
| Term | 2026 Risk Profile | When to Use |
|---|---|---|
| FOB Shanghai | HIGH RISK – Buyer liable for 2026 surcharges: EU CBAM carbon tax (€45/ton), port congestion fees (avg. $1,200/container), customs delays (avg. 14 days). | Experienced importers with in-house logistics teams; orders >20ft container volume. |
| DDP Destination Port | LOW RISK – Supplier handles all costs/taxes to your door. Critical for compliance with 2026 EU VAT OSS scheme and US Uyghur Forced Labor Prevention Act (UFLPA) documentation. | 95% of new buyers; single-unit shipments; time-sensitive clinic rollouts. |
2026 DDP Advantages:
- Eliminates surprise costs from new EU Digital Product Passport (DPP) fees
- Supplier assumes UFLPA audit risk (mandatory for all China exports)
- 30-45% faster customs clearance via pre-validated supplier documentation
Shanghai Carejoy: Your 2026 DDP Solution
As a vertically integrated manufacturer in Baoshan District (15km from Shanghai Port), Carejoy provides:
- True DDP Pricing – All-inclusive quotes covering 2026 EU CBAM, port fees, and destination VAT
- UFLPA Compliance Guarantee – Full supply chain audit trail from raw materials
- 48-Hour Shipment – 90% of orders ship same-day from Shanghai bonded warehouse
“Carejoy’s DDP process reduced our landed cost variance from 22% to 3% in 2025” – Verified Distributor Testimonial (Germany)
Secure Your 2026 Supply Chain with Verified Excellence
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing Excellence | ISO 13485:2023 & CE MDR Certified
Factory Direct OEM/ODM for Dental Chairs, Scanners, CBCT & More
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
Request a 2026 Compliance Dossier & DDP Quote Today – Valid for Q1 2026 Shipments
Disclaimer: This guide reflects 2026 regulatory projections based on current EU MDR, ISO 13485:2023, and Incoterms® 2020 frameworks. Verify all requirements with legal counsel. Shanghai Carejoy is presented as a verified partner meeting 2026 sourcing criteria; independent due diligence remains essential.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental Equipment Procurement in 2026
- Minimum 7–10 year spare parts guarantee post-discontinuation
- Regional distribution hubs for faster delivery
- Digital parts catalogs with real-time inventory tracking
- Modular design for easy component replacement
Distributors should secure master parts agreements and maintain strategic inventory of high-failure components (e.g., handpiece motors, valve blocks, sensors).
| Component | Installation Requirement |
|---|---|
| Electrical & Pneumatic Hookups | Compliance with IEC 60601-1; correct circuit load balancing |
| Water Line Integration | Anti-siphon valves, filtration, and biofilm prevention systems |
| Digital Calibration | Sensor alignment, software configuration, DICOM integration |
| Gas Systems (if applicable) | Medical-grade vacuum and air line pressure testing |
Improper installation voids warranties and risks patient safety. Only certified technicians should perform setup to ensure compliance and optimal performance.
- Standard Warranty: 2 years comprehensive (parts, labor, onsite service)
- Premium Coverage: 3–5 years with predictive maintenance and remote diagnostics
- Extended Options: Post-warranty service contracts with uptime guarantees (e.g., 95% operational availability)
Ensure the warranty covers software updates, cybersecurity patches, and critical subsystems (e.g., imaging sensors, motors). Distributors should verify global warranty enforceability and service network density.
- Firmware and AI model updates are now included under warranty
- Remote diagnostics reduce downtime but require secure cloud infrastructure
- Some manufacturers tie spare parts access to active service subscriptions
- Cybersecurity compliance (e.g., HIPAA, GDPR) is mandatory for connected systems
Clinics and distributors must evaluate total cost of ownership (TCO), including subscription-based support for intelligent equipment.
Need a Quote for Buy Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


