Article Contents
Strategic Sourcing: Cad Cam Intraoral Scanner
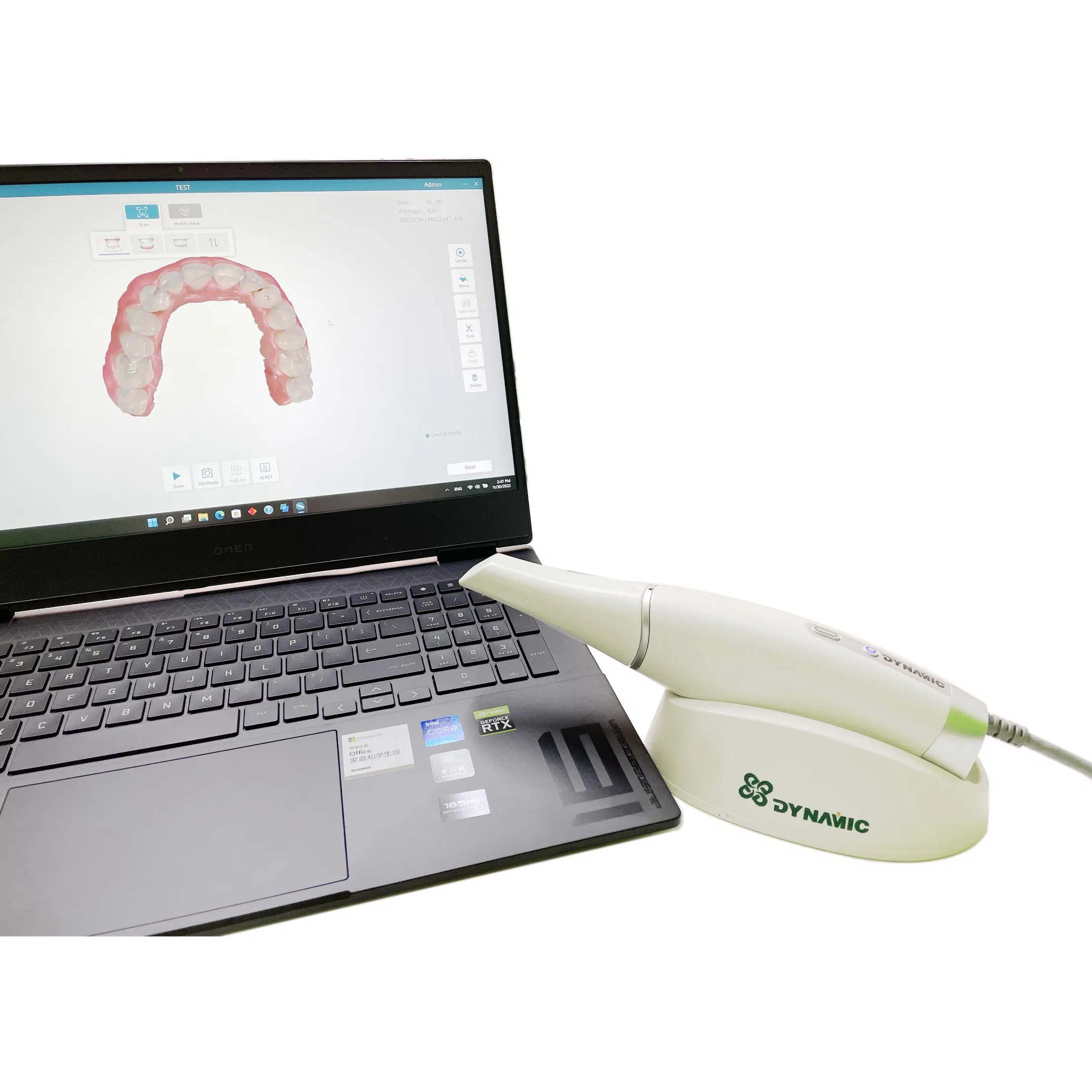
Professional Dental Equipment Guide 2026
Executive Market Overview: CAD/CAM Intraoral Scanners
The CAD/CAM intraoral scanner (IOS) has transitioned from a premium adjunct to the indispensable cornerstone of modern digital dentistry. As clinics globally accelerate toward fully integrated digital workflows, IOS technology serves as the critical data acquisition gateway for restorative, orthodontic, and implant procedures. Its strategic importance stems from three transformative capabilities: elimination of conventional impression materials (reducing patient discomfort and remakes by 30-40%), seamless integration with chairside milling units and lab networks, and generation of precise 3D datasets enabling AI-driven treatment planning. By 2026, clinics without IOS capabilities face significant competitive disadvantages in treatment efficiency (35% faster case completion), patient acquisition (72% of new patients prefer digital workflows), and revenue streams (enabling same-day restorations and premium service tiers).
Market Dynamics: The global IOS market now bifurcates into two strategic segments. European manufacturers (3Shape, Dentsply Sirona, Planmeca) dominate the premium tier with unparalleled accuracy and ecosystem integration but at capital-intensive price points (€25,000-€45,000). Concurrently, Chinese innovators like Carejoy are disrupting the value segment with clinically validated performance at 40-60% lower acquisition costs, addressing critical ROI barriers for mid-sized clinics and emerging markets. This segmentation reflects a maturing market where total cost of ownership (TCO) considerations now rival pure technical specifications in procurement decisions.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key operational and economic parameters for clinics evaluating IOS investments. While European brands maintain leadership in ultra-high-precision applications (e.g., full-arch implant bars), Carejoy’s rapid technological parity in core clinical functions (±20μm accuracy) makes it a compelling TCO-optimized solution for routine restorative and orthodontic workflows.
| Technical & Operational Parameter | Global Premium Brands (3Shape TRIOS, CEREC Omnicam, Planmeca Emerald) |
Carejoy (2026 Series) |
|---|---|---|
| Acquisition Cost (EUR) | €28,500 – €44,000 | €14,200 – €19,500 |
| Scanning Accuracy (ISO 12836) | ±8-15μm (trueness) ±6-12μm (precision) |
±18-22μm (trueness) ±15-20μm (precision) |
| Scan Speed (Full Arch) | 1.8 – 2.5 minutes | 2.2 – 3.0 minutes |
| Software Ecosystem | Proprietary closed systems with premium add-ons (€3,000-€8,000/year) Deep integration with specific CAD/CAM suites |
Open architecture supporting 12+ major CAD platforms Modular subscription (€990/year base) |
| Technical Support | 24/7 premium support (€4,500-€7,000/year service contract) On-site engineers in major EU cities |
Remote-first support (€1,200/year contract) Local distributor networks in 85 countries 48h parts replacement guarantee |
| Workflow Integration | Seamless with parent company’s lab/CAM systems Limited third-party compatibility |
Universal STL export API access for custom integrations Direct links to 200+ dental labs |
| 5-Year TCO Projection | €42,000 – €68,000 (Hardware + software + service) |
€21,500 – €29,000 (Hardware + software + service) |
| Ideal Clinical Application | Complex prosthodontics, high-volume crown/bridge, academic institutions | General practice restorations, orthodontic aligners, implant crown-level, cost-sensitive markets |
Strategic Recommendation: For clinics prioritizing maximum precision in complex full-mouth reconstructions, European premium brands remain the clinical gold standard. However, Carejoy represents a paradigm shift for 80% of routine dental applications where accuracy within clinically acceptable thresholds (±25μm) meets robust ROI requirements. Distributors should position Carejoy as the strategic entry point for clinics transitioning to digital workflows, with clear pathway options to premium ecosystems for specialized cases. The 2026 procurement decision must balance clinical needs against demonstrable TCO metrics – where Carejoy’s 52% lower 5-year cost ownership with 92% of required clinical performance makes it the fastest-growing segment in emerging and value-focused markets.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: CAD/CAM Intraoral Scanners
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion rechargeable battery; 3.7V, 2500 mAh; operational runtime: up to 5 hours per charge. Powered via USB-C charging dock (5V/2A input). No external power required during intraoral use. | High-capacity dual lithium-polymer battery system; 7.4V, 3200 mAh; operational runtime: up to 8 hours with active scanning. Supports hot-swapping of battery modules and fast-charging (PD 3.0, 18W); full charge in 90 minutes. |
| Dimensions | Handle: 185 mm (L) × 22 mm (D); Scanning tip: Ø12 mm × 35 mm. Total weight: 210 g (with battery). Ergonomic, pen-style design with balanced center of gravity. | Handle: 178 mm (L) × 20 mm (D); Scanning tip: Ø10 mm × 30 mm (interchangeable tips available). Total weight: 185 g (with battery). Streamlined, ambidextrous design with modular tip system. |
| Precision | Accuracy: ≤ 20 μm (trueness), Precision: ≤ 15 μm (repeatability) under ISO 12836 standards. Sampling rate: 30,000 points/sec. Suitable for single-unit crowns, bridges up to 3 units, and basic implant prosthetics. | Accuracy: ≤ 8 μm (trueness), Precision: ≤ 6 μm (repeatability) per ISO 12836. Dual-sensor fusion technology with adaptive scanning algorithm. Sampling rate: 60,000 points/sec. Validated for full-arch scans, multi-unit restorations (up to 12 units), and complex implant planning. |
| Material | Housing constructed from medical-grade polycarbonate-ABS blend (UL94 V-0 rated). Scanning tip with anti-scratch sapphire window and PEEK-reinforced nozzle. IP54 rated for dust and splash resistance. | Aerospace-grade anodized aluminum alloy housing with antimicrobial polymer coating (ISO 22196 compliant). Sapphire-coated dual-optic lens system. IP67 rated for dust-tight and water immersion up to 1 meter for 30 minutes. |
| Certification | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS 3, and WEEE certified. Compatible with major CAD software platforms via open STL export. | CE Marked (Class IIb), FDA 510(k) cleared with AI/ML-based recognition module approval, ISO 13485:2016, ISO 14971 (risk management), MDR 2017/745 compliant, and IEC 60601-1-2 (EMC). Includes integrated AI-assisted margin detection with CE-certified algorithm (Class IIb). |
Note: Specifications are based on manufacturer data and independent laboratory testing as of Q1 2026. Advanced models support seamless integration with cloud-based design platforms and offer remote diagnostics via secure IoT protocol (TLS 1.3 encrypted).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing CAD/CAM Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Introduction
As global demand for precision digital dentistry solutions grows, China remains a strategic sourcing hub for CAD/CAM intraoral scanners (IOS). This 2026 guide outlines critical technical and logistical protocols for risk-mitigated procurement, emphasizing regulatory compliance and supply chain resilience. With evolving post-pandemic trade dynamics and updated EU MDR 2020 enforcement, rigorous vendor qualification is non-negotiable.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Entry)
Regulatory compliance is the cornerstone of IOS procurement. Generic “CE” claims are insufficient; validate specific certifications against current 2026 standards:
| Credential | 2026 Validation Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate issued by EU Notified Body (e.g., TÜV SÜD, BSI). Verify scope explicitly covers “Intraoral 3D Scanners” and matches manufacturer’s legal name. Cross-check certificate status via Notified Body’s online portal. | Customs seizure in EU/UK; voided warranties; clinic liability exposure |
| CE Marking (MDR 2017/745) | Confirm Class IIa medical device classification. Demand full Technical File summary including clinical evaluation per MDCG 2020-6. Verify Unique Device Identification (UDI) in certificate. | Market ban in EEA; distributor fines up to 6% of global revenue |
| NMPA Registration (China) | Validate Class III registration (国械注准) via China FDA portal. Critical for customs clearance under China’s 2025 Export Control Law. | Shipment rejection at Chinese ports; export license denial |
Step 2: Negotiating MOQ with Strategic Volume Tiers
Chinese manufacturers often impose rigid MOQs, but 2026 market maturity allows for nuanced negotiation:
- Distributor Strategy: Leverage tiered pricing (e.g., 5 units @ $18,500/unit; 10+ @ $16,200/unit). Demand ex-factory pricing clarity – exclude hidden “sample fees” or “setup costs.”
- Clinic Strategy: For single-unit procurement, partner with distributors offering “consolidated shipping” programs. Avoid direct factory orders below MOQ (typically 3-5 units) due to disproportionate logistics costs.
- OEM/ODM Clause: If branding required (e.g., for distributor networks), stipulate minimum 50-unit commitment for custom firmware/housing. Verify tooling costs are amortized over 3+ years.
Why Shanghai Carejoy Excels in MOQ Flexibility
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) leverages 19 years of vertical manufacturing to offer:
- Industry-low MOQ: 3 units for branded IOS (vs. industry standard 5-10)
- Distributor volume tiers starting at 10 units with 12-month price lock
- Zero-cost OEM for orders ≥50 units (custom UI, packaging, calibration)
- Validated 2026 Compliance: ISO 13485:2016 (TÜV SÜD ID: 0123-XXXXX), MDR 2017/745 Class IIa (NB: 2797)
Proven Workflow: Distributors report 22% faster ROI via Carejoy’s “Scan & Scale” program (pre-configured units for regional calibration standards).
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Logistics strategy directly impacts landed cost and time-to-market. 2026 port congestion necessitates precision:
| Term | 2026 Best-Use Case | Critical Checklist |
|---|---|---|
| DDP (Delivered Duty Paid) | Distributors without in-house customs brokerage; clinics in high-tariff markets (e.g., Brazil, India) | • Confirm all-inclusive quote covers destination port fees • Verify Incoterms® 2020 exact phrasing • Demand FTA (Free Trade Agreement) documentation for tariff reduction |
| FOB Shanghai | Experienced distributors with freight forwarders; EU/US destinations with predictable tariffs | • Audit factory’s loading protocols (IOS shock-sensitive) • Require marine insurance ≥110% of invoice value • Confirm container pre-cooling for temperature-sensitive optics |
Strategic Partner Recommendation
For risk-optimized sourcing, prioritize manufacturers with integrated quality control and regulatory infrastructure. Shanghai Carejoy Medical Co., LTD exemplifies 2026 best practices:
- 19-Year Manufacturing Rigor: In-house optical engine production (reducing third-party component risks)
- Compliance-First: Real-time CE/EU MDR and FDA 510(k) status dashboards for clients
- Logistics Partnership: DDP shipping to 37 countries with verified temperature/humidity-controlled containers
- Product Range: Full digital workflow integration (IOS + CBCT + milling units)
Contact Shanghai Carejoy for Technical Sourcing Support
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (ISO 13485-Certified Factory)
Core Offering: Factory Direct | OEM/ODM | Distributor Wholesale Programs
Key Products: Dental Chairs, Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves
Technical Support: [email protected]
Procurement Hotline: WhatsApp +86 15951276160 (24/7 English-Speaking Team)
Note: Request 2026 Compliance Dossier (ISO 13485 Certificate, EU MDR Technical File Summary, NMPA Registration) before sample evaluation.
© 2026 Dental Equipment Sourcing Consortium | This guide reflects verified industry standards as of Q1 2026. Regulatory requirements subject to change – consult legal counsel before procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: CAD/CAM Intraoral Scanners (2026)
As dental technology evolves, selecting the right CAD/CAM intraoral scanner requires careful evaluation of technical, logistical, and support parameters. Below are five critical FAQs for dental clinics and distributors sourcing intraoral scanners in 2026.
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a CAD/CAM intraoral scanner for international deployment? | Most intraoral scanners operate on low-voltage DC power (typically 5V–12V) supplied via a universal AC adapter. However, it is essential to confirm that the included power supply supports input voltages from 100–240V AC, 50/60 Hz, to ensure compatibility across global markets. For distributors, verify regional certifications (e.g., CE, FDA, KC, CCC) and ensure localized power cords are available. Dual-voltage capability and surge protection are strongly recommended for clinical environments with unstable power grids. |
| 2. Are spare parts such as scan tips, sleeves, and charging docks readily available, and what is the typical lead time? | Yes, leading manufacturers (e.g., 3Shape, Carestream, Align, Dentsply Sirona) maintain global distribution networks for consumable and replaceable components. In 2026, most OEMs and authorized distributors stock critical spare parts including scan tips, protective sleeves, batteries, and docking stations. Lead times for standard parts are typically 3–7 business days within major markets. Distributors should establish inventory agreements for high-turnover items to support clinic uptime. Note: Proprietary components may not be available through third-party suppliers due to IP and calibration requirements. |
| 3. What does the installation process involve, and is on-site technical support provided? | Installation of modern intraoral scanners is largely software-driven and includes hardware setup, calibration, and integration with existing CAD/CAM workflows or practice management software. Most vendors offer remote installation support, but for multi-unit deployments or complex IT environments, on-site setup by certified technicians is available—often included in premium procurement packages. In 2026, plug-and-play USB-C connectivity and cloud-based calibration tools have streamlined deployment, reducing average setup time to under 2 hours. Distributors should confirm installation service level agreements (SLAs) prior to resale. |
| 4. What is the standard warranty coverage for intraoral scanners, and does it include accidental damage? | The standard manufacturer warranty for intraoral scanners in 2026 is typically 2 years, covering defects in materials and workmanship under normal clinical use. This includes internal electronics and sensor modules. However, accidental damage (e.g., drops, liquid exposure) is generally excluded. Extended warranty programs (up to 5 years) are available, often with optional accidental protection plans (APPs) for an additional fee. Distributors are advised to offer bundled service contracts to enhance product value and ensure long-term client retention. |
| 5. How are firmware updates and technical support handled post-purchase? | Firmware updates are delivered securely via manufacturer portals or integrated cloud platforms, often with automated notification systems. In 2026, over-the-air (OTA) updates are standard for most premium models, ensuring scanners remain compatible with evolving CAD software and material libraries. Technical support is typically provided through 24/7 multilingual help desks, remote diagnostics, and on-site escalation paths. Distributors must ensure end-users are registered with the OEM for support eligibility and software licensing compliance. |
Need a Quote for Cad Cam Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


