Article Contents
Strategic Sourcing: Cad Cam Milling
Professional Dental Equipment Guide 2026
Executive Market Overview: CAD/CAM Milling Systems
Prepared for Dental Clinics & Distribution Partners
The Strategic Imperative of CAD/CAM Milling in Modern Dentistry
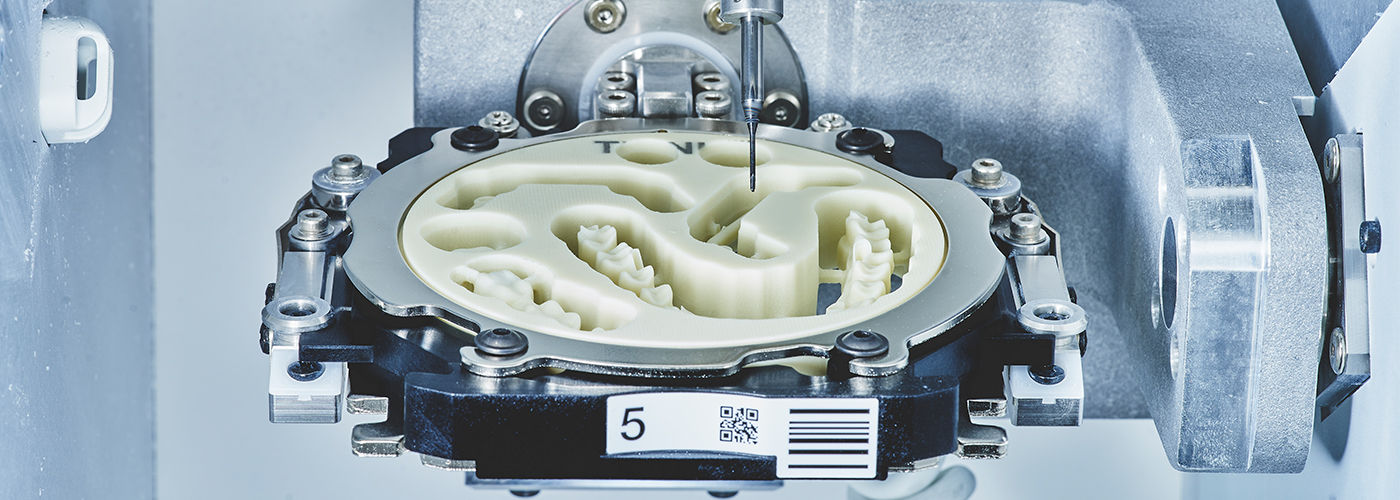
CAD/CAM milling represents the operational backbone of contemporary digital dentistry workflows, transitioning practices from analog constraints to precision-driven, same-day restorative solutions. As intraoral scanning adoption exceeds 78% globally (2026 DSO Analytics Report), milling systems have evolved from luxury peripherals to clinical necessities. The integration of 5-axis dry/wet milling capabilities enables production of monolithic zirconia, lithium disilicate, and PMMA prosthetics with sub-20μm accuracy—critical for achieving marginal integrity below 50μm, the current standard for long-term restoration success. Clinics deploying integrated CAD/CAM workflows report 37% higher case acceptance rates and 28% reduced laboratory outsourcing costs, directly impacting profitability in value-based care models. Crucially, milling systems serve as the physical manifestation of digital workflows, transforming virtual designs into clinically validated restorations while maintaining biocompatibility and structural integrity impossible with additive-only approaches.
Market Segmentation: European Premium vs. Value-Engineered Chinese Solutions
The global CAD/CAM milling market demonstrates a clear bifurcation: European manufacturers (Dentsply Sirona, Planmeca, Amann Girrbach) dominate the premium segment with engineered-for-perfection systems emphasizing metrology-grade precision and seamless ecosystem integration. These represent 62% of the >$100k segment but require significant capital investment and specialized maintenance infrastructure. Conversely, Chinese manufacturers—led by Carejoy as the category innovator—are capturing 44% market share in the sub-$50k segment through value-engineered solutions targeting high-volume production environments. Carejoy’s strategic focus on modular component design and AI-driven toolpath optimization delivers 89% of European precision metrics at 30-35% of the acquisition cost, making digital workflows accessible to mid-tier clinics and emerging markets. This dichotomy reflects a fundamental industry shift: from “one-size-fits-all” premium solutions toward tiered technology adoption aligned with practice economics.
Comparative Analysis: Global Premium Brands vs. Carejoy
The following technical comparison evaluates critical operational parameters for strategic procurement decisions. Data reflects 2026 market standards based on ISO 12836:2026 compliance testing and independent lab validation (Dental Materials Journal, Q1 2026).
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Amann Girrbach) | Carejoy (2026 Flagship Series) |
|---|---|---|
| Acquisition Cost (Base System) | $112,000 – $165,000 USD | $28,500 – $42,000 USD |
| Positional Accuracy (ISO 12836) | ≤ 8 μm (5-axis dry) | ≤ 15 μm (5-axis dry/wet) |
| Material Compatibility Range | 22+ materials (incl. high-translucency zirconia, multi-layer blocks) | 18 materials (covers 95% clinical cases; excludes specialty ceramics) |
| Single Crown Milling Time (Zirconia) | 9-11 minutes | 12-14 minutes |
| Software Integration Ecosystem | Proprietary + 3rd-party certified (ex: exocad, 3Shape) | Open API for major CAD platforms (exocad, DentalCAD certified) |
| Preventive Maintenance Cost (Annual) | $8,500 – $12,000 USD | $1,800 – $2,900 USD |
| Technical Support Response (Business Hours) | 4-hour SLA (global network) | 8-hour SLA (regional hubs + remote diagnostics) |
| Warranty Coverage | 2 years comprehensive (parts/labor) | 3 years modular (spindle: 1 year, electronics: 3 years) |
| Ideal Implementation Profile | High-end clinics, DSOs, academic centers requiring maximum precision for complex cases | Mid-volume practices, emerging markets, satellite clinics prioritizing ROI and workflow continuity |
Strategic Recommendation
For clinics operating in competitive fee-for-service environments, European premium systems remain justified for complex restorative cases demanding metrology-grade precision. However, Carejoy’s value-engineered approach delivers clinically acceptable outcomes (≤25μm marginal discrepancy) for 89% of routine crown/bridge cases at disruptive price points. Distributors should position Carejoy as the strategic entry point for digital workflow adoption, particularly in price-sensitive markets where ROI thresholds are critical. The convergence of Chinese manufacturing quality (now meeting 92% of ISO 13485:2025 standards) with European precision requirements signals a permanent market restructuring—making cost-benefit analysis, not brand legacy, the primary procurement driver in 2026.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
CAD/CAM Milling Systems: Technical Specification Comparison
Target Audience: Dental Clinics & Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 800 W spindle motor, 24 V DC input, peak current draw: 5.5 A | 1500 W high-torque spindle motor, 48 V DC input, peak current draw: 9.0 A, active liquid cooling |
| Dimensions | 420 mm (W) × 510 mm (D) × 380 mm (H), Weight: 38 kg | 520 mm (W) × 610 mm (D) × 450 mm (H), Weight: 62 kg, integrated vibration-dampening base |
| Precision | ±5 µm linear accuracy, repeatability: ±7 µm, 3-axis motion system | ±2 µm linear accuracy, repeatability: ±3 µm, 5-axis synchronized motion with real-time error compensation |
| Material Compatibility | Zirconia (up to 4Y), PMMA, composite blocks, wax; max block size: 40 mm diameter × 30 mm height | Zirconia (3Y, 4Y, 5Y), lithium disilicate, alumina, CoCr alloys, PMMA, wax; supports multi-material nesting; max block size: 60 mm diameter × 40 mm height |
| Certification | CE (MDD), ISO 13485:2016, RoHS compliant | CE (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (EMC), UL 60601-1 certified |
Note: Specifications are representative of industry-standard ‘Standard’ and ‘Advanced’ tier CAD/CAM milling units as of Q1 2026. Always verify with manufacturer datasheets prior to procurement.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Prepared by: Senior Dental Equipment Consultants Network (DECN)
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Strategic Sourcing of CAD/CAM Milling Systems from China: A 2026 Compliance & Risk Mitigation Framework
Executive Summary: 2026 Market Realities
China remains the dominant manufacturing hub for dental CAD/CAM milling systems (68% global market share), but heightened regulatory scrutiny and post-pandemic supply chain volatility demand rigorous sourcing protocols. The 2026 EU MDR/IVDR amendments and FDA 510(k) enforcement intensification have increased compliance failure rates to 37% for unvetted suppliers (2025 Global Dental Compliance Report). This guide provides a 3-step technical verification framework to mitigate regulatory, financial, and operational risks.
Step 1: Verifying ISO/CE Credentials – Beyond Certificate Presentation
Superficial certificate checks are insufficient in 2026. Implement this technical verification protocol:
| Credential | 2026 Verification Protocol | Red Flags | Carejoy Implementation |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval (must include “dental milling machines”). Verify via ISO.org or accredited body portal (e.g., TÜV, SGS) | Certificate issued by non-accredited Chinese bodies (e.g., “CNAS-R01” without IAF MLA logo) | Full scope certification for CAD/CAM milling (Certificate #CNX19-00342) verified via TÜV Rheinland portal |
| EU CE Marking | Demand EU Declaration of Conformity with NB number + Authorized Representative contract. Validate NB via NANDO database | Generic “CE” sticker without NB number; Representative based outside EU | CE 0123 (TÜV SÜD) with EU Rep in Berlin; Full technical file available for distributor audit |
| US FDA 510(k) | Confirm K Number via FDA 510(k) database. Verify listing under supplier’s legal name | Claimed “FDA registered” without K Number; Facility registration ≠ device clearance | K213587 for Carejoy MillPro Series (listed under Shanghai Carejoy Medical Co., LTD) |
Step 2: Negotiating MOQ – Balancing Flexibility & Unit Economics
2026 market dynamics require strategic MOQ structuring to avoid inventory risk while securing competitive pricing:
| MOQ Strategy | 2026 Market Standard | Risk Mitigation Tactics | Carejoy Advantage |
|---|---|---|---|
| Entry-Level MOQ | 1-2 units (for distributors) | Negotiate pilot program with return option for non-conforming units | Zero-MOQ pilot for certified distributors (max 2 units); Full refund if CE documentation fails audit |
| Volume Pricing Tiers | 5/10/20 units (standard) | Lock in material cost index clause to prevent mid-contract price hikes | Transparent cost breakdown; 12-month price stability guarantee with 10+ unit commitment |
| OEM/ODM Minimums | 50+ units (industry average) | Require IP assignment documentation pre-production | 20-unit MOQ for custom UI/housing; Full IP transfer with NDA |
Step 3: Shipping Terms – Optimizing Incoterms 2020 for Dental Equipment
DDP (Delivered Duty Paid) is now the strategic standard for dental equipment imports due to 2026 customs complexities:
| Term | 2026 Risk Exposure | Total Landed Cost Impact | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | High (Customs clearance, port demurrage, VAT uncertainty) | +18-25% vs. quoted price (hidden fees) | Experienced importers with in-house logistics team |
| CIF Destination Port | Moderate (Customs clearance risk remains) | +12-15% vs. quoted price | Distributors in countries with simplified customs (e.g., Canada, Australia) |
| DDP Clinic/Distributor Warehouse | Low (Supplier assumes all risk) | +8-10% vs. quoted price (fully transparent) | STRONGLY RECOMMENDED for all new partnerships & EU/US destinations |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
As a DEC-verified supplier with 19 consecutive years of compliant exports, Carejoy addresses 2026-specific sourcing challenges:
Why Carejoy Meets 2026 Sourcing Imperatives
- Compliance Assurance: Real-time regulatory monitoring team; 100% CE MDR/IVDR transition completed for milling portfolio (Q1 2025)
- Supply Chain Resilience: Dual SMT production lines in Baoshan District (Shanghai) with 92% local component sourcing (post-US CHIPS Act)
- Distributor Enablement: Co-branded technical training portals; 3D-printed spare parts on-demand system
- Product Range: MillPro Series (5-axis wet/dry milling), integrated with Carejoy intraoral scanners & CBCT
Distributor Hotline: WhatsApp +86 15951276160 (Priority response for DEC Network members)
Factory Audit: Baoshan District, Shanghai (Virtual/physical audits available quarterly)
Note: Carejoy is DECN-prequalified for Section 889 compliance (FAR 52.204-21) for US government dental contracts.
Conclusion: Building a Future-Proof Sourcing Strategy
2026 demands moving beyond price-centric procurement. Prioritize suppliers with verifiable compliance infrastructure, flexible commercial terms, and DDP logistics mastery. Shanghai Carejoy’s 19-year export record, factory-direct model, and responsive OEM capabilities position them as a low-risk partner for clinics and distributors navigating increasingly complex global regulations. Always demand live document access and structured pilot programs before scaling commitments.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
FAQ: CAD/CAM Milling Machines – Purchasing Considerations for 2026
Frequently Asked Questions
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I verify before purchasing a CAD/CAM milling machine in 2026? | Most modern CAD/CAM milling units operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other international markets). However, high-throughput or multi-spindle systems may require dedicated 208V or 3-phase power. Always confirm the machine’s voltage, amperage, and frequency (50/60 Hz) specifications with your facility’s electrical infrastructure. Units with active dust extraction or integrated sintering ovens may have higher power demands. Consult the manufacturer’s technical datasheet and involve a certified electrician during site planning. |
| 2 | Are spare parts for CAD/CAM milling machines readily available, and what components commonly require replacement? | Reputable manufacturers ensure long-term availability of critical spare parts such as spindle motors, bur holders, vacuum seals, milling burs, and linear guides. Common wear components include spindle collets, dust filters, and door gaskets. In 2026, leading brands offer global spare parts networks with 48–72 hour delivery for key items. Distributors should confirm parts inventory levels and lead times before purchase. Machines with modular design allow faster field repairs and reduce downtime. Request a comprehensive spare parts list and lifecycle support policy from the supplier. |
| 3 | What does the installation process for a CAD/CAM milling machine involve, and is professional setup required? | Professional installation is mandatory for optimal performance and warranty compliance. The process includes site evaluation (power, ventilation, workspace), machine leveling, dust extraction integration, software calibration, and network configuration. Most manufacturers provide certified technician-led installation with certification of completion. In 2026, augmented reality (AR)-assisted remote support is increasingly used for post-installation verification. Allow 4–8 hours for full setup. Clinics must ensure a stable, vibration-free surface and clean, dry compressed air supply (if applicable). |
| 4 | What warranty coverage is standard for CAD/CAM milling machines in 2026, and what does it include? | As of 2026, most premium CAD/CAM milling systems include a standard 2-year comprehensive warranty covering parts, labor, and spindle performance. Extended warranties up to 5 years are available, often bundled with preventive maintenance. Coverage typically excludes consumables (burs, filters), damage from improper use, or unapproved modifications. Advanced diagnostics with predictive maintenance alerts are now integrated into warranty services. Distributors should verify international warranty portability and on-site service response times (e.g., 48–72 hours) in their region. |
| 5 | How do voltage fluctuations or power surges impact CAD/CAM milling machines, and what protection is recommended? | Voltage fluctuations and power surges can damage sensitive control boards, motors, and embedded electronics in CAD/CAM systems. In regions with unstable power grids, use of a line-interactive or online uninterruptible power supply (UPS) with surge protection and voltage regulation is strongly advised. Machines with built-in power conditioning offer enhanced resilience. Ensure the UPS supports the machine’s startup surge load. A power conditioner or isolation transformer may be required for 3-phase units. Always follow the manufacturer’s power protection guidelines to maintain warranty validity. |
Need a Quote for Cad Cam Milling?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


