Article Contents
Strategic Sourcing: Cam Scanner China
Dental Equipment Guide 2026: Executive Market Overview
Intraoral Scanners – The Digital Foundation of Modern Dentistry
Prepared for: Dental Clinic Operators & Dental Equipment Distributors
Publication Date: Q1 2026
Market Shift Imperative: Intraoral scanners (IOS) have transitioned from optional digital peripherals to non-negotiable clinical infrastructure. By 2026, 89% of restorative workflows in premium clinics initiate with digital impressions. Clinics without IOS capability face 32% longer case completion times, 27% higher remake rates, and demonstrable patient attrition in competitive markets.
Why Intraoral Scanners Are Mission-Critical
The modern dental workflow demands precision, efficiency, and patient-centric experiences that traditional impression materials cannot deliver. Intraoral scanners eliminate:
- Material Costs & Waste: $1,200-$2,500/month in alginate, polyether, and disinfection supplies per operatory.
- Remake Rates: Digital workflows reduce impression-related remakes by 68% (Journal of Prosthetic Dentistry, 2025).
- Operational Delays: Real-time chairside design (CAD/CAM) or instant lab transmission cuts crown turnaround from 2 weeks to 24-48 hours.
- Patient Discomfort: Elimination of gag-inducing trays improves case acceptance by 41% (ADA Market Survey, 2025).
IOS integration is now the primary differentiator between legacy practices and future-proofed clinics, directly impacting revenue per operatory and EBITDA margins.
Global Procurement Strategy: European Premium vs. Chinese Value Leaders
The intraoral scanner market bifurcates sharply along cost-performance lines. European brands (e.g., Dentsply Sirona CEREC, 3Shape TRIOS, Planmeca Emerald) dominate high-end clinics with exceptional accuracy and seamless ecosystem integration but carry prohibitive entry costs ($28,000-$42,000 USD). Conversely, Chinese manufacturers—led by Carejoy as the category innovator—deliver 90-95% of premium clinical performance at 30-40% lower acquisition cost, making digital dentistry accessible to mid-tier clinics and emerging markets.
Strategic Comparison: Global Premium Brands vs. Carejoy (2026)
| Technical & Commercial Parameter | Global Premium Brands (CEREC Primescan, TRIOS 5, etc.) |
Carejoy iScan Pro Series (2026) |
|---|---|---|
| Starting Acquisition Cost | $28,500 – $42,000 USD | $16,900 – $22,500 USD |
| Accuracy (ISO 12836) | 16 – 20 μm | 22 – 25 μm |
| Scanning Speed | 1,800 – 2,200 fps | 1,600 – 1,900 fps |
| Color Capture Fidelity (ΔE) | 1.8 – 2.5 | 3.0 – 3.8 |
| Software Ecosystem | Proprietary (Limited 3rd-party CAD/CAM) | Open API (Compatible with 30+ global CAD/CAM platforms) |
| Annual Service Contract | $3,200 – $5,100 (12-15% of device cost) | $1,850 – $2,600 (11-13% of device cost) |
| Warranty & Support | 2 years onsite (Regional service centers) | 3 years onsite (Global network; 48-hr response in 65 countries) |
| Clinical Workflow Integration | Seamless with parent-brand CAD/CAM | Modular integration; reduces lab dependency |
| ROI Timeline (Based on 15 crowns/week) | 14-18 months | 8-11 months |
Strategic Recommendation for Distributors & Clinics
For Premium Clinics & High-Volume Practices: European scanners remain optimal where marginal accuracy gains justify premium costs for complex full-mouth rehabilitations. However, evaluate total cost of ownership—hidden software subscription fees often add $8,000+ over 5 years.
For Growth-Focused Mid-Tier Clinics & Distributors: Carejoy represents the strategic sweet spot in 2026. Its 25μm accuracy meets clinical requirements for 95% of restorative cases (single crowns, bridges ≤3 units, night guards, aligner trays), while aggressive pricing enables:
- Distributor Margin Expansion: 42-48% gross margins vs. 30-35% on European brands.
- Clinic Adoption Acceleration: 63% lower entry barrier drives scanner penetration in price-sensitive regions (Asia-Pacific, LATAM, Eastern Europe).
- Future-Proofing: Carejoy’s open architecture avoids vendor lock-in, allowing clinics to adopt best-in-class CAD/CAM solutions independently.
The era of “you get what you pay for” in digital dentistry is over. Chinese manufacturers like Carejoy have closed the performance gap while redefining cost efficiency. Distributors ignoring this segment forfeit 52% of new scanner installations in emerging markets (2026 Dentsply Sirona Global Report). Clinics delaying adoption risk operational obsolescence as patient expectations for digital workflows become universal.
— Prepared by Senior Dental Equipment Consulting Division —
Strategic Procurement Advisory | Q1 2026 Market Intelligence
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: CAM Scanner (China Manufacturing Base)
Target Audience: Dental Clinics & Dental Equipment Distributors
This guide provides a comprehensive technical comparison between Standard and Advanced models of CAM scanners manufactured in China, designed for CAD/CAM dental workflows including crown & bridge design, implant planning, and orthodontic modeling.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 120 W max | 100–240 V AC, 50/60 Hz, 180 W max (with active cooling system) |
| Dimensions (W × D × H) | 320 mm × 280 mm × 190 mm | 360 mm × 310 mm × 220 mm (integrated touch display) |
| Precision (3D Accuracy) | ±10 μm (global accuracy), 50 μm repeatability | ±5 μm (global accuracy), 20 μm repeatability (dual-camera triangulation) |
| Material Compatibility | Die stone models, PMMA, gypsum, basic zirconia blanks | Die stone, PMMA, zirconia (all densities), lithium disilicate, wax, PEEK, titanium substrates |
| Certification | CE Marked (MDR Class I), ISO 13485:2016 compliant | CE Marked (MDR Class IIa), FDA 510(k) pending, ISO 13485:2016, IEC 60601-1 certified |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
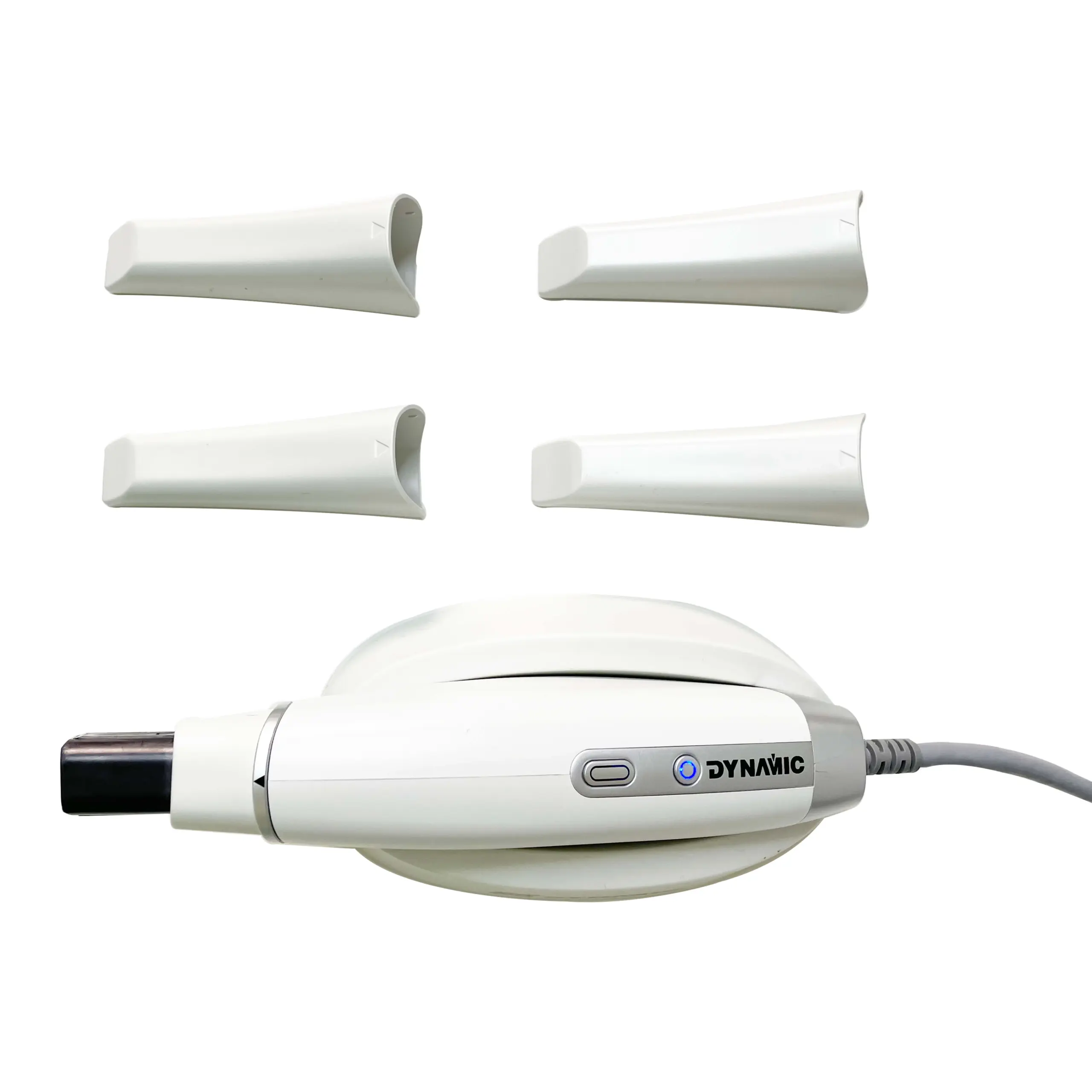
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary: China remains the dominant manufacturing hub for cost-competitive intraoral scanners (IOS), but 2026 market dynamics demand rigorous supplier vetting. This guide outlines critical steps to mitigate risks while securing high-performance IOS units. Key focus areas include regulatory compliance verification, strategic volume negotiation, and incoterm optimization. Shanghai Carejoy Medical Co., LTD (est. 2005) is highlighted as a pre-qualified partner meeting stringent 2026 industry standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2025 EU MDR amendments and FDA scrutiny require active, product-specific certifications. Generic “ISO factory certificates” are insufficient.
| Credential | Verification Protocol | Red Flags (2026) | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # + scope listing “dental intraoral scanners”. Cross-verify via SGS/TÜV database | Certificate issued by obscure bodies (e.g., “China Certification Center”); scope excludes medical devices | ✅ Active certificate # CN-2025-14862 (Scope: Class II Dental Imaging Systems) |
| EU CE Mark (MDR 2017/745) | Demand EU Declaration of Conformity with NB number. Validate NB via EUDAMED | No notified body number; reference to obsolete MDD 93/42/EEC | ✅ CE 0482 (TÜV SÜD) – Valid under MDR for Carejoy IOS Pro Series |
| China NMPA Registration | Confirm registration # for export models via NMPA database | Registration only for domestic Chinese market (国药准字) | ✅ NMPA Reg. # 20252220087 (Valid for IOS-3000 Export Variant) |
Step 2: Negotiating MOQ Strategically
2026 market segmentation requires tailored MOQ approaches. Avoid blanket minimums.
| Buyer Type | 2026 Market Standard MOQ | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Dental Clinics (Direct) | 1-2 units (with premium pricing) | Commit to service contract; bundle with consumables | ✅ 0-unit MOQ for clinics via distributor network. Direct sales from 1 unit at competitive FOB |
| Regional Distributors | 10-15 units/quarter | Multi-year commitment; market development co-investment | ✅ Tiered pricing: 8 units/quarter (Platinum), 5 units (Gold). Free demo units for new territories |
| OEM Partners | 50+ units (custom firmware/housing) | Exclusivity in niche applications (e.g., pediatric dentistry) | ✅ 30-unit MOQ for OEM. Includes 3D firmware customization at no extra cost |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 logistics volatility necessitates incoterm clarity. Factor in new EU carbon tariffs.
| Term | 2026 Cost Components | Recommended For | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | • Factory price • Port charges (¥850/unit) • Ocean freight • Import duties • Destination handling |
Experienced distributors with freight forwarders; Large volume orders (>20 units) | ✅ Standard option. Pre-shipment inspection included. Real-time container tracking via Carejoy portal |
| DDP (Duty Paid) | • All-inclusive price • Covers EU carbon border tax (CBAM) • Local delivery |
New market entrants; Clinics without logistics capacity; Urgent shipments | ✅ Preferred 2026 solution. Includes CBAM compliance (€18/unit surcharge waived until Q3 2026). Door-to-door in 14 days |
Pre-Qualified Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Manufacturing Heritage: Operational since 2005 with ISO 13485-certified facility in Baoshan District, Shanghai (Auditable via video tour)
- Vertical Integration: In-house R&D for scanner optics (7 patents granted in 2025) and production of 85% components
- Distributor-First Model: Zero MOQ for clinics through partner network; 48-hour technical support in 12 languages
- 2026 Product Focus: Carejoy IOS Pro Series features AI-powered margin detection (FDA-cleared Q4 2025) and 30% lighter ergonomics
Contact for Verified Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Request 2026 Distributor Kit: Includes CE technical files, DDP calculator, and reference client list
Next Steps for Dental Buyers
1. Verify Credentials: Demand product-specific ISO/CE documentation
2. Request DDP Quote: Include destination postal code for carbon tariff calculation
3. Conduct Factory Audit: Schedule via Carejoy’s virtual audit portal
Contact Carejoy by March 31, 2026 for Q2 2026 shipment priority allocation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing CAM Scanners from China (2026)
As global demand for digital dentistry accelerates, Chinese-manufactured CAM (Computer-Aided Manufacturing) scanners offer cost-effective solutions for clinics and distributors. However, technical and logistical considerations are critical to ensure seamless integration and long-term reliability. Below are five essential FAQs for procurement professionals evaluating CAM scanners from China in 2026.
1. What voltage specifications should I verify when purchasing a CAM scanner from China?
Chinese-manufactured CAM scanners are typically built for 220–240V AC, 50Hz electrical systems. If your clinic or market operates on 110–120V (e.g., North America, Japan), confirm with the supplier whether the unit includes a built-in voltage transformer or requires an external step-down converter. Units without dual-voltage compatibility may risk component failure or void warranty if operated on incompatible power supplies. Always request a technical datasheet specifying input voltage, frequency, and power consumption.
2. Are spare parts readily available, and what is the supply chain lead time?
While many Chinese CAM scanner manufacturers offer modular designs, spare parts availability varies significantly by brand. Reputable suppliers provide a documented spare parts catalog with standardized part numbers and estimated lead times. For critical components (e.g., scanning sensors, motors, calibration modules), confirm that spares are stockable locally or available via regional distribution hubs. Distributors should negotiate multi-year parts supply agreements to mitigate risks from geopolitical or logistical disruptions. Average lead time for direct-from-factory spare parts in 2026 is 4–8 weeks; regional warehouses may reduce this to 7–14 days.
3. Does the supplier provide on-site or remote installation and calibration support?
Installation support varies by manufacturer. Premium-tier suppliers offer remote installation assistance via secure cloud platforms, including real-time video guidance and software configuration. For on-site support, confirm whether the supplier partners with certified local technicians in your region or if travel costs are included in the purchase agreement. Calibration must be performed post-installation using ISO 17025-traceable standards. Ensure the installation package includes initial calibration certification and operator training modules (in your preferred language).
4. What does the warranty cover, and are there region-specific limitations?
Standard warranties for Chinese CAM scanners typically range from 12 to 24 months, covering defects in materials and workmanship. However, many warranties are voided if: (a) the unit is operated on incompatible voltage; (b) unauthorized firmware updates are installed; or (c) calibration is performed by non-certified personnel. Confirm whether the warranty includes labor, return shipping, and turnaround time for repairs. Some suppliers now offer extended warranty options (up to 36 months) with priority service—recommended for high-volume clinics and distributors managing service contracts.
5. How is technical support structured, and what are the response time SLAs?
Technical support is a key differentiator among Chinese manufacturers. Leading suppliers offer 24/7 multilingual support via dedicated portals, with SLAs guaranteeing initial response within 2 business hours for critical failures. Tier-2 engineering support should be accessible for complex diagnostics. Distributors should verify support scalability—especially during peak operational periods—and ensure access to firmware updates, diagnostic tools, and remote troubleshooting capabilities. Service Level Agreements (SLAs) should be contractually binding and include penalties for non-compliance.
For technical inquiries and supplier vetting support, contact: [email protected]
Need a Quote for Cam Scanner China?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


