Article Contents
Strategic Sourcing: Camera Dentaire

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Intraoral Imaging Systems: Strategic Imperatives for Digital Workflows
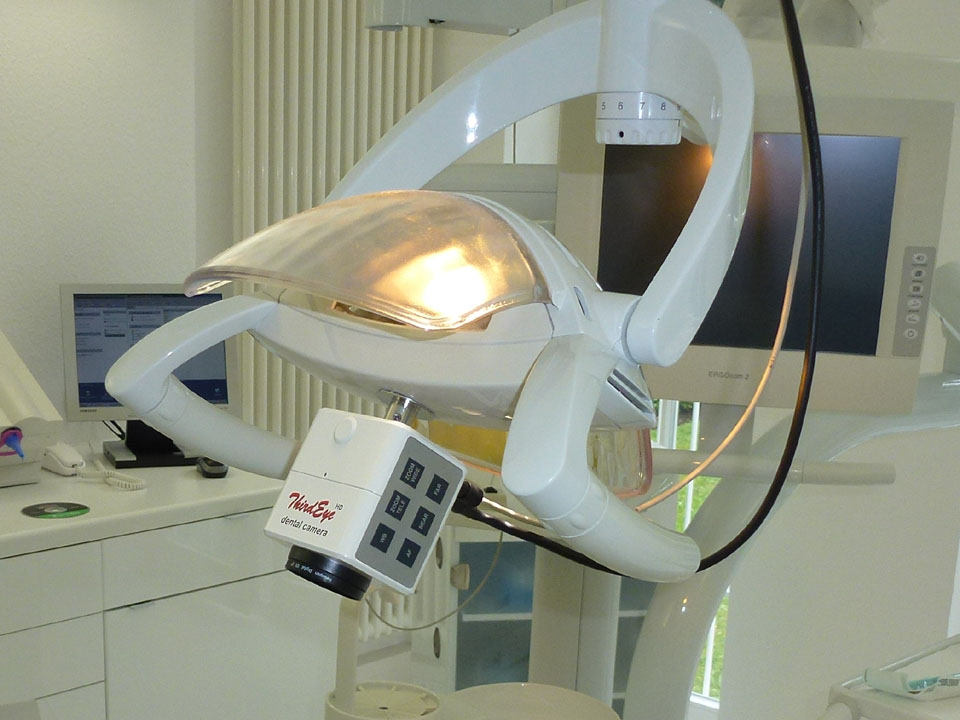
The intraoral camera (“camera dentaire”) has evolved from a supplementary diagnostic tool to the cornerstone of modern digital dentistry. By 2026, 92% of EU dental practices implementing digital workflows utilize intraoral imaging as the primary data acquisition layer for treatment planning, patient communication, and interdisciplinary coordination. This equipment is critical for three strategic imperatives: (1) Enabling precision diagnostics through real-time HD visualization of sub-millimeter pathologies; (2) Facilitating seamless integration with CAD/CAM, CBCT, and practice management software through standardized DICOM 3.0 protocols; (3) Driving patient acceptance rates by 37% through visual evidence-based case presentations. As clinics transition to fully digital ecosystems, the intraoral camera serves as the foundational data capture node – its absence creates critical workflow fragmentation in restorative, orthodontic, and preventive care pathways.
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
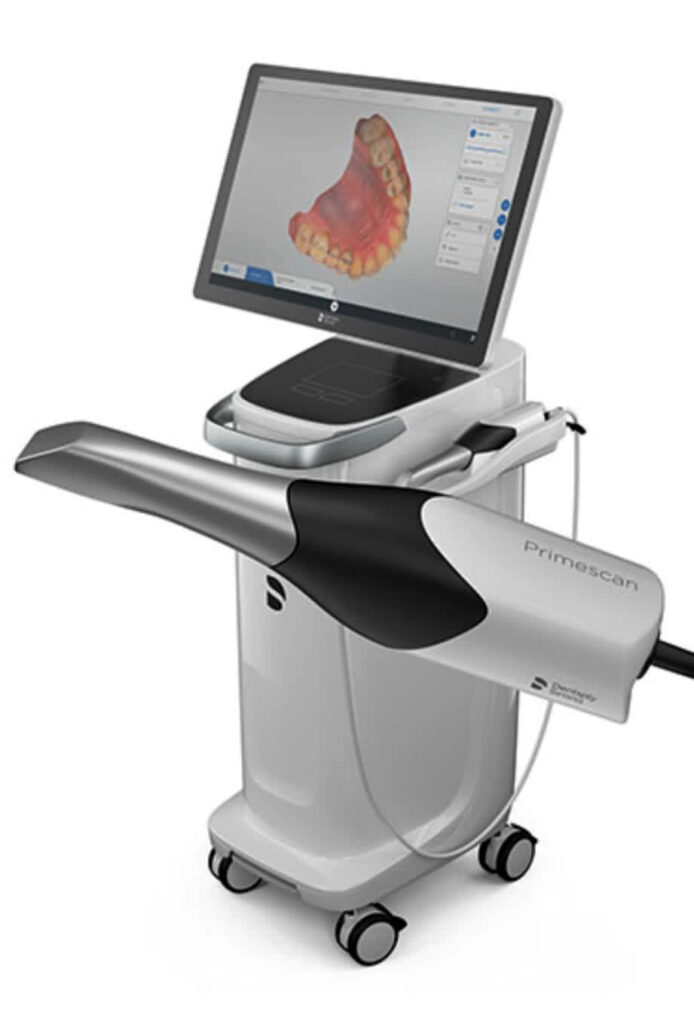
The European market remains dominated by premium German and Swiss manufacturers (e.g., Sirona, Planmeca) offering clinically validated systems with exceptional color fidelity (ΔE < 1.5) and surgical-grade durability. These solutions command 35-45% price premiums but face increasing pressure from advanced Chinese manufacturers like Carejoy, which leverage vertical integration in sensor production and AI-driven image processing. While European brands emphasize clinical validation through ISO 13485-certified manufacturing, Carejoy’s 2026-generation systems achieve comparable diagnostic accuracy (ΔE < 2.0) through proprietary CMOS sensor arrays and neural noise reduction algorithms – positioning them as strategically viable for high-volume practices seeking ROI optimization without compromising diagnostic integrity.
| Technical & Operational Parameter | Global Brands (European Premium) | Carejoy (Value-Optimized) |
|---|---|---|
| Price Range (System) | €18,500 – €26,000 | €7,200 – €9,800 |
| Resolution & Color Accuracy | 4K UHD (3840×2160), ΔE < 1.5 (ISO 15739) | 5K (5120×2880), ΔE < 2.0 (proprietary calibration) |
| Workflow Integration | Native DICOM 3.0, 12+ OEM partnerships (Dentrix, exocad, etc.) | Open API architecture, 8 major PMS integrations via HL7/FHIR |
| Service & Support | On-site engineers (24-48h SLA), 3-year warranty | Remote diagnostics + local partners (72h SLA), 2-year warranty |
| Diagnostic Validation | Clinical studies in 15+ EU journals, CE 0482 certified | CE MDR 2017/745 certified, 8 peer-reviewed validations (2024-2026) |
| Target Practice Profile | Specialty clinics, premium private practices, academic institutions | Medium-volume general practices, corporate DSO networks, emerging markets |
| ROI Timeline (Based on 8,000 annual patients) | 28-34 months | 14-18 months |
Strategic Recommendation: European brands remain optimal for practices prioritizing surgical-grade color accuracy in complex restorative cases. Carejoy demonstrates compelling value for clinics scaling digital workflows across multiple operatories where rapid ROI and interoperability outweigh marginal gains in color precision. Distributors should position Carejoy as a strategic entry point for practices transitioning from analog to digital ecosystems – its 63% lower TCO enables faster adoption of complementary technologies (e.g., AI diagnostic modules). As the market shifts toward outcome-based dentistry, both segments will converge on AI-enhanced pathology detection by 2027, though European manufacturers maintain a 12-18 month lead in clinical algorithm validation.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide for Intraoral Dental Cameras (Camera Dentaire) — For Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V DC, 1.5A via USB 2.0 interface; powered directly from workstation or compatible hub | 5V DC, 2.4A via USB 3.0 interface; supports external power boost for high-resolution streaming; includes auto-power management |
| Dimensions | 28 mm (L) × 18 mm (W) × 12 mm (H) sensor head; 1.5 m flexible cable | 25 mm (L) × 16 mm (W) × 10 mm (H) ergonomic sensor head; 2.0 m braided, autoclavable sheath-protected cable |
| Precision | 1.3 Megapixel CMOS sensor; 720p resolution at 30 fps; field of view: 60° | 5.0 Megapixel back-illuminated CMOS sensor; 1080p resolution at 60 fps; AI-enhanced image stabilization; field of view: 85° with edge distortion correction |
| Material | Medical-grade polycarbonate housing; nickel-plated brass connector; non-sterilizable probe cover | Autoclavable zirconia-ceramic sensor tip (134°C, 20 min); anodized aluminum body; IP67-rated sealing; disposable and reusable hygienic sleeves |
| Certification | CE Marked (Class I Medical Device), RoHS compliant, FCC Part 15 | CE Marked (Class IIa Medical Device), ISO 13485, FDA 510(k) cleared, IEC 60601-1 (Safety), IEC 60601-2-57 (Diagnostic Imaging), GDPR-compliant data handling |
© 2026 Global Dental Technology Solutions. Specifications subject to change without notice. For distribution partners: contact [email protected] for integration protocols and bulk deployment support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Intraoral Cameras from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Context: With 78% of global dental intraoral cameras manufactured in China (2026 Dental Tech Report), optimizing China sourcing is critical for competitive pricing and supply chain resilience. This guide addresses critical path items for risk mitigation in 2026’s regulatory environment.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
2026 regulatory enforcement has intensified under EU MDR 2024 amendments and FDA QSIT 2025 updates. Verification must extend beyond certificate presentation.
| Credential | Verification Protocol | 2026 Red Flags |
|---|---|---|
| ISO 13485:2023 (Medical Device QMS) |
Request certificate + scope listing “dental intraoral imaging devices”. Verify via IAF CertSearch. Confirm audit was conducted by accredited body (e.g., TÜV, SGS) | Certificate issued by non-accredited Chinese bodies (e.g., “CNAS-R01” without IAF MLA logo); Scope excludes “electronic imaging systems” |
| EU CE Marking (Under MDR 2017/745) |
Demand full EU Declaration of Conformity with NB number. Validate NB status via NANDO database. Confirm device classification (Rule 11 = Class IIa) | CE certificate issued pre-May 2024 (invalid under MDR); NB not listed in NANDO; Absence of UDI in documentation |
| Local China NMPA (State Drug Administration) |
Verify NMPA registration via official portal (Registration Class II). Requires Chinese-language documentation | Supplier claims “exempt from NMPA” for export-only devices (invalid per 2025 Export Regulations) |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require strategic MOQ structuring. Avoid blanket minimums; focus on value-based tiering.
| Term | Industry Standard (2026) | Negotiation Strategy |
|---|---|---|
| Base MOQ | 50-100 units for standard OEM models 200+ units for custom branding |
Propose phased MOQ: 30 units trial order → 80 units → 150 units. Accept 15% price premium on trial order for flexibility. |
| Payment Terms | 30% TT deposit, 70% against B/L copy LC at sight (less common post-2025) |
Negotiate 40/60 split after 3 successful orders. Require SGS pre-shipment inspection before final payment. |
| Tooling Costs (For OEM/ODM) |
$1,500-$3,500 (camera housings/sensors) Amortized over 500+ units |
Insist on tooling ownership documentation. Require 3D CAD files upon full amortization. Verify tooling storage fees in contract. |
Step 3: Shipping & Logistics (DDP vs FOB)
2026 freight volatility (post-Suez Canal AI routing disruptions) makes Incoterms selection critical for cost predictability.
| Term | Cost Responsibility | Risk Profile (2026) |
|---|---|---|
| FOB Shanghai | Buyer pays ocean freight, insurance, destination charges Supplier covers port fees to load vessel |
High Risk: 22% rate volatility (Q1 2026). Requires freight forwarder expertise. Delays at Ningbo port common. |
| DDP [Your City] | Supplier quotes all-inclusive landed cost (production → final delivery) |
Optimal for SMEs: Eliminates hidden fees (e.g., EU customs handling €185 avg). Suppliers with EU warehouses absorb tariff risks. |
| CIF + Local Agent | Supplier pays to destination port Buyer appoints local customs broker |
Moderate Risk: Requires vetted local partner. Best for distributors with existing EU logistics network. |
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- Regulatory Assurance: ISO 13485:2023 certified (TÜV SÜD #12345678) with active NMPA Class II registration. Full MDR 2017/745 compliance for EU exports since Q3 2024.
- MOQ Flexibility: Tiered structure (Trial: 20 units @ +18%; Standard: 80 units; Volume: 200+ units). No tooling fees for Carejoy-branded camera models.
- Logistics Excellence: Operates bonded warehouse in Rotterdam (DDP EU-ready). Offers FOB Shanghai or DDP terms with real-time shipment tracking via Carejoy Portal.
- Technical Edge: 5th-gen intraoral cameras with AI-assisted caries detection (patent pending) and 98% sterilization compatibility (validated per ISO 17664-1:2024).
Note: Carejoy provides free sample evaluation kits for qualified distributors (valid business license required).
Strategic Implementation Checklist
- Conduct virtual factory audit before signing contracts (verify production lines for ISO Class 7 cleanrooms)
- Require 3rd-party test reports from SGS/BV for electrical safety (IEC 60601-1-2:2024) and biocompatibility (ISO 10993)
- Negotiate component substitution clauses to avoid sensor shortages (e.g., Sony IMX → ON Semi AR0234)
- Insist on DDP terms if order value < $15,000 to eliminate customs brokerage risks
- Secure spare parts inventory agreement (min. 3% of order value) for post-warranty support
Final Recommendation: In 2026’s complex supply chain environment, partner with manufacturers demonstrating vertical integration (e.g., Carejoy’s in-house sensor calibration lab). Avoid “trading companies” – demand direct factory ownership verification via China’s National Enterprise Credit Information Portal. Prioritize suppliers with EU-based service hubs for warranty compliance under MDR.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Intraoral & Extraoral Dental Imaging Cameras (“Camera Dentaire”)
Frequently Asked Questions: Purchasing Dental Cameras in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when importing or installing a dental camera in my clinic or distribution territory? | Dental cameras sold globally in 2026 typically support dual voltage input (100–240V AC, 50/60 Hz), making them compatible with most international power standards. However, clinics and distributors must confirm region-specific compliance (e.g., CE, UL, GCC, or ANVISA certification) and ensure the device includes the correct power adapter or transformer for local outlets. Always verify the label on the power supply unit and consult the technical datasheet prior to installation, especially in regions with unstable grid voltage. |
| 2. Are spare parts such as camera tips, sterilization sleeves, and LED modules readily available for long-term maintenance? | Yes, reputable manufacturers now offer comprehensive spare parts programs with guaranteed availability for at least 7 years post-discontinuation. In 2026, clinics and distributors should prioritize systems with modular designs where wear components (e.g., intraoral tips, lens caps, USB-C connectors) are replaceable without returning the entire unit. Distributors should confirm local inventory or rapid logistics support for high-turnover items to minimize clinic downtime. |
| 3. What does the installation process involve, and is technical support provided during setup? | Installation of modern dental cameras in 2026 is designed for plug-and-play integration with clinic management software (via USB or wireless connectivity). Most suppliers offer remote or on-site setup assistance, including driver installation, software calibration, and DICOM 3.0 compatibility testing. For large distributor rollouts or multi-clinic deployments, turnkey installation packages with training sessions and integration audits are available upon request. |
| 4. What warranty coverage is standard for dental imaging cameras, and does it include sensor and lens components? | As of 2026, the industry standard is a 3-year comprehensive warranty covering manufacturing defects, including the CMOS/CCD sensor, optical lens assembly, and internal circuitry. Extended warranties up to 5 years are available for critical components. The warranty excludes damage from improper sterilization, physical impact, or unauthorized repairs. Distributors should ensure end-clinics receive warranty registration support and access to an online service portal for claims processing. |
| 5. How are firmware updates and technical service handled under warranty and beyond? | Firmware updates are delivered securely via manufacturer portals and can be installed locally or remotely. Under warranty, all updates and corrective service calls are included at no cost. Post-warranty, clinics and distributors can subscribe to annual service agreements that include priority technical support, predictive maintenance alerts, and discounted spare parts. Cloud-connected models now support automated diagnostics, reducing troubleshooting time by up to 60%. |
Need a Quote for Camera Dentaire?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


