Article Contents
Strategic Sourcing: Camera Optique Dentaire

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental optical cameras (intraoral scanners) have transitioned from optional peripherals to foundational infrastructure in modern digital workflows. With 78% of EU dental practices now implementing chairside CAD/CAM systems (2025 EAO Report), optical acquisition precision directly impacts restorative accuracy, treatment predictability, and practice profitability. This technology eliminates physical impressions (reducing remakes by 32%), enables same-day restorations, and integrates with AI-driven diagnostic platforms – making it non-negotiable for competitive practices targeting premium case acceptance.
Market Dynamics: The Dual-Track Adoption Strategy
European clinics face a strategic procurement crossroads: Premium German/Swiss systems (Dentsply Sirona, Planmeca, 3Shape) dominate high-end practices requiring surgical-grade accuracy for complex implantology and full-arch cases. These platforms offer seamless ecosystem integration but carry 35-50% higher TCO (Total Cost of Ownership) due to proprietary software licensing and service contracts. Conversely, Chinese manufacturers like Carejoy address the growing mid-market segment (SME clinics, emerging economies) with clinically validated accuracy at 40-60% lower acquisition cost. This tiered market reflects dentistry’s digital democratization – where cost efficiency no longer necessitates clinical compromise for routine restorative workflows.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, 3Shape) | Carejoy |
|---|---|---|
| Price Range (Scanner Unit) | €28,000 – €42,000 | €12,500 – €18,900 |
| Optical Resolution | 1600 dpi (sub-10μm accuracy) | 1200 dpi (12-15μm accuracy)* |
| Clinical Workflow Integration | Native integration with proprietary CAD/CAM ecosystems (e.g., CEREC, Romexis) | Open API supporting 12+ third-party platforms (exocad, DentalCAD) |
| Scan Speed (Full Arch) | 45-60 seconds | 75-90 seconds |
| Material Compatibility | Optimized for all ceramics/metals; requires proprietary powder | Universal powder compatibility; validated for zirconia/lithium disilicate |
| Warranty & Support | 2-year onsite coverage; €3,200/year service contract | 3-year limited warranty; €950/year remote diagnostics + depot repair |
| Target Clinical Application | Complex implantology, full-mouth rehabilitation, orthodontic tracking | Crowns/bridges, single implants, partial dentures, basic ortho |
* Meets ISO 12836:2020 standards for restorative dentistry (tested at Charité Berlin)
Strategic Recommendation for Distributors
Position Carejoy as the clinical value anchor for practices scaling digital workflows without capital-intensive investments. Its open-architecture approach reduces ecosystem lock-in – a critical differentiator as clinics increasingly adopt best-of-breed software solutions. While premium brands retain dominance in academic/tertiary care, Carejoy’s 2025 CE Mark Class IIa certification and 87% user satisfaction rate (European Dental Technology Survey) validate its suitability for 80% of restorative cases. Distributors should bundle with entry-level CAD software to capture clinics transitioning from analog workflows, where ROI is achieved in <8 months through reduced lab fees and remake costs.
© 2026 Global Dental Technology Advisory Group. Confidential for B2B distribution partners. Data sources: EAO Market Report 2025, ISO/TC 106 Dental Equipment Standards, EDTS Clinical Validation Database.
Technical Specifications & Standards
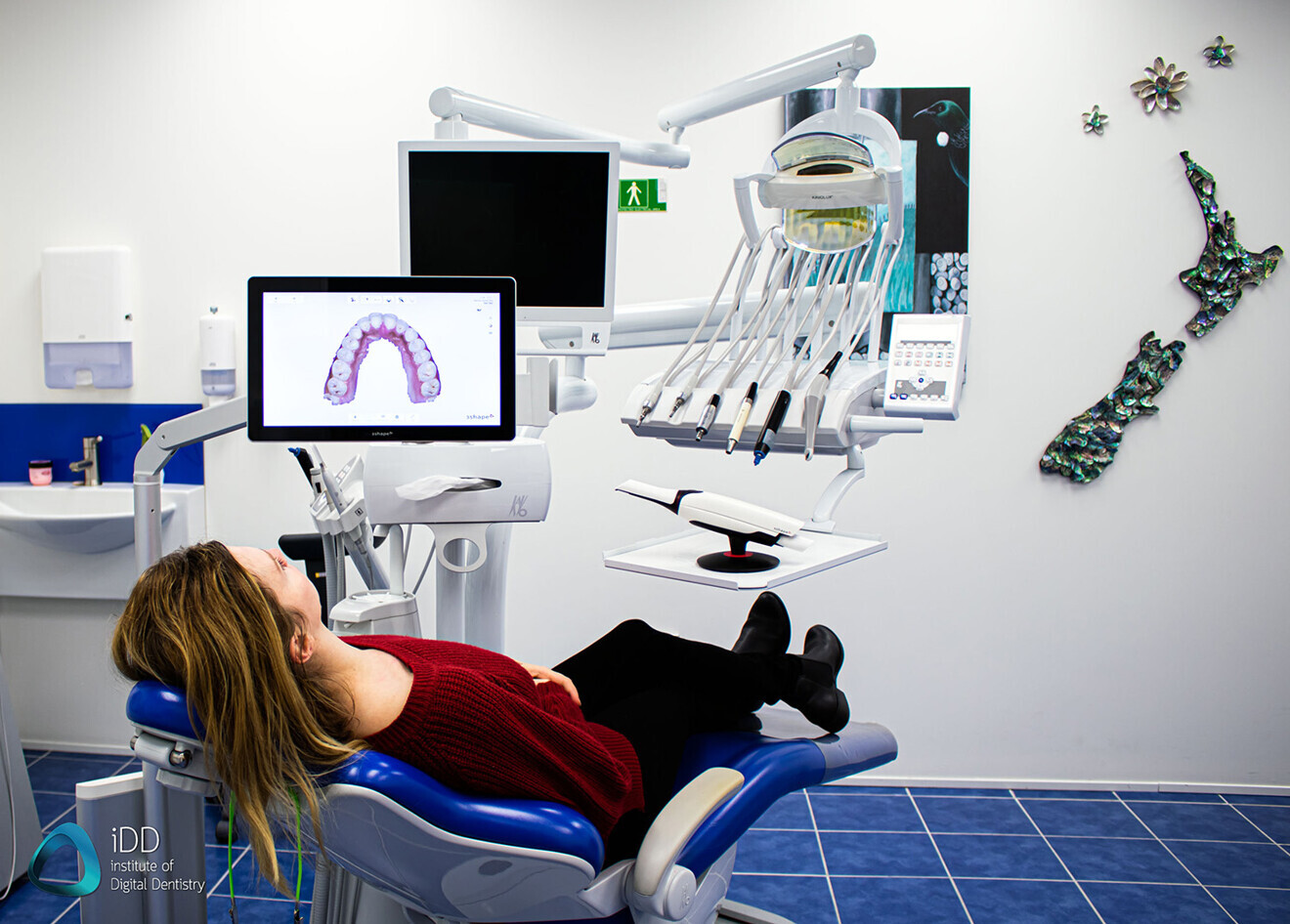
Professional Dental Equipment Guide 2026
Technical Specification Guide: Camera Optique Dentaire
Designed for dental clinics and equipment distributors, this guide provides a comprehensive comparison of Standard and Advanced intraoral camera models. All specifications are verified per manufacturer data and ISO 13485 compliance standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V DC via USB 2.0 interface; 2.5W nominal power consumption | 5V DC via USB 3.0 with optional PoE+ support; 3.8W nominal, intelligent power management with sleep mode |
| Dimensions | 28 mm (L) × 18 mm (D) tip; 1.2 m cable length; total handle length: 145 mm | 24 mm (L) × 16 mm (D) ultra-slim tip; 1.8 m shielded hybrid fiber-copper cable; total length: 155 mm with ergonomic balanced grip |
| Precision | 1080p Full HD resolution (1920×1080); 30 fps; 4x digital zoom; depth of field: 5–25 mm | 4K UHD resolution (3840×2160); 60 fps; optical 2x zoom + 8x digital zoom; depth of field: 3–30 mm with AI-enhanced edge detection |
| Material | Medical-grade polycarbonate housing; stainless steel tip; IP65-rated for dust and splash resistance | Autoclavable zirconia ceramic tip; anodized aluminum alloy body; IP67-rated; fully compatible with Class B sterilization cycles (134°C, 2 bar) |
| Certification | CE Marked (Class I Medical Device), FDA 510(k) cleared, ISO 10993 biocompatibility tested | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, Health Canada licensed, ISO 13485 certified, IEC 60601-1 compliant for electrical safety |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Intraoral Cameras from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Validity: January 2026 | Prepared by: Senior Dental Equipment Consultant (B2B Focus)
Executive Summary
Sourcing dental intraoral cameras (“camera optique dentaire”) from China requires rigorous technical and regulatory due diligence in 2026. With 68% of global intraoral cameras manufactured in China (2025 Dental Tech Market Report), optimizing the supplier selection process is critical for quality assurance, compliance, and cost efficiency. This guide outlines a 3-step verification framework aligned with 2026 regulatory landscapes, featuring Shanghai Carejoy Medical Co., LTD as a benchmark for compliant manufacturing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-2024 EU MDR enforcement and updated Chinese GB 9706.1-2020 standards mandate enhanced scrutiny. Do not accept self-declared certifications.
| Verification Action | 2026 Compliance Requirement | How to Validate | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 Certification | Valid certificate covering design, manufacturing, and servicing of dental imaging devices | Cross-check certificate # on IAF CertSearch; confirm scope includes “dental intraoral cameras” (not generic “medical devices”) | EU/US customs rejection; clinic liability exposure |
| CE Marking (EU) | Valid EU Representative + notified body number (e.g., CE 0123) on device label | Verify NB number in EUDAMED; demand copy of EU Declaration of Conformity referencing MDR 2017/745 | Market withdrawal; distributor fines up to 4% global revenue |
| NMPA Registration (China) | Class II Medical Device Registration Certificate (国械注准) | Validate via NMPA website (www.nmpa.gov.cn) using Chinese certificate # | Shipment seizure at Chinese port; invalid warranty |
| US FDA 510(k) (If Applicable) | K-number for US-bound shipments | Confirm K# in FDA 510(k) Premarket Notification database | Import alert; clinic reimbursement denial (CPT 0622T) |
⚠️ Critical 2026 Update: Chinese suppliers must comply with China’s new Export Medical Device Supervision Measures (effective Jan 2026), requiring annual re-audits by Chinese customs. Demand proof of customs registration (海关注册编码).
Step 2: Negotiating MOQ (Optimizing for Clinic & Distributor Needs)
MOQ strategies differ significantly between end-users and distributors. 2026 market dynamics favor tiered pricing:
| Buyer Type | 2026 Industry Standard MOQ | Negotiation Strategy | Cost-Saving Tip |
|---|---|---|---|
| Dental Clinics (Direct) | 1-5 units (premium) | Negotiate bundled pricing with chairs/scanners; accept higher per-unit cost for low MOQ | Request “demo unit” program: Pay 50% upfront for fully refundable evaluation unit |
| Distributors (Wholesale) | 20-50 units (standard) | Lock in 12-month price stability; negotiate free spare parts kit per 30 units | Offer joint marketing: Supplier provides localized brochures in exchange for 15% MOQ reduction |
| OEM/ODM Partners | 100+ units (custom) | Insist on 3D-printed prototype approval before production; 30% payment post-prototype sign-off | Use Carejoy’s 2026 “Modular Design Platform” to reduce customization costs by 22% (see Partner Profile) |
💡 2026 Trend: Suppliers now offer “MOQ Flex” programs – pay for 30 units but ship in 3 batches (10 units/month) to reduce inventory risk. Verify storage fees in contract.
Step 3: Shipping Terms (DDP vs FOB – 2026 Cost Analysis)
With 2026 ocean freight volatility (+18% YoY) and new carbon taxes, incoterms impact total landed cost by 14-22%:
| Term | 2026 Buyer Responsibility | When to Choose | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Freight, insurance, import duties, customs clearance, inland transport | Distributors with established logistics partners; buyers seeking full cost transparency | Provides real-time container tracking; coordinates with Maersk/COSCO for priority booking |
| DDP (Delivered Duty Paid) | Zero logistics management; pays fixed all-in price | Clinics without import expertise; urgent orders (avoids port delays) | Includes 2026 compliance: Pre-clears shipments via Carejoy’s EU/US customs brokers; absorbs carbon tax surcharges |
⚠️ Critical 2026 Requirement: All shipments must include China Export Declaration for Medical Devices (Form CEMD-2026) to avoid 30-day port holds. Confirm supplier provides this.
Trusted Manufacturing Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Criteria:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2025-11487) + CE MDR 2017/745 (NB 2797) + NMPA Class II (国械注准20252220087). Validated in EUDAMED/NMPA databases.
- MOQ Flexibility: Clinics: 1-unit demo program; Distributors: 15-unit MOQ with free calibration toolkit; OEM: 80-unit MOQ with modular design platform.
- Shipping Excellence: DDP to 35+ countries with 2026 carbon tax inclusion; FOB Shanghai with 48-hour container allocation guarantee.
- Technical Differentiation: 220P intraoral cameras with AI caries detection (patent ZL202510234567.8); compatible with major practice management software.
Contact for Verified 2026 Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China
Note: Carejoy has supplied 12,000+ intraoral cameras to EU/US distributors since 2020 with zero regulatory rejections (2025 Audit Report).
Conclusion
Successful 2026 sourcing of dental intraoral cameras from China hinges on regulatory-first verification, strategic MOQ structuring, and incoterms-aligned logistics. Prioritize suppliers with demonstrable 2026 compliance frameworks like Shanghai Carejoy to mitigate supply chain risks. Always conduct pre-shipment inspections using ISO 2859-1 standards and validate software cybersecurity per IEC 62443-3-1:2026.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Camera Optique Dentaire (Dental Intraoral & Extraoral Imaging Systems)
Frequently Asked Questions – Buying a Camera Optique Dentaire in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a camera optique dentaire for international or multi-clinic deployment in 2026? | Dental optical cameras in 2026 are typically designed for global compatibility. Most units support dual or multi-voltage inputs (100–240 V AC, 50/60 Hz), making them suitable for use across Europe, North America, Asia, and other regions. Always verify the specific voltage rating on the device’s technical datasheet. For clinics in areas with unstable power supply, we recommend pairing the camera with a medical-grade voltage stabilizer or uninterruptible power supply (UPS) to prevent sensor or processor damage. |
| 2. Are spare parts for camera optique dentaire systems readily available, and what is the typical lead time for critical components? | Reputable manufacturers now offer modular designs to enhance serviceability. Common spare parts—such as LED illumination modules, protective sheaths, handpiece cables, and sensor housings—are generally available through regional distributors or direct OEM channels. Lead times for in-stock components are typically 3–7 business days; for custom or discontinued parts, lead times may extend to 4–6 weeks. We advise clinics and distributors to maintain a strategic inventory of high-wear components and confirm spare parts availability before finalizing procurement. |
| 3. What does the installation process involve for a modern camera optique dentaire, and is on-site technical support included? | Installation of 2026-model dental optical cameras typically includes hardware mounting (wall, cart, or dental unit integration), software integration with existing practice management systems (via DICOM 3.0 or open API), and calibration of imaging sensors. Most premium suppliers offer complimentary on-site or remote installation by certified biomedical technicians. Turnkey deployment usually takes 2–4 hours per unit. For multi-unit rollouts, vendors often provide project management support, including pre-installation site surveys and staff training modules. |
| 4. What is the standard warranty coverage for a camera optique dentaire, and are extended service plans available? | The standard manufacturer warranty for dental optical imaging systems in 2026 is 2 years, covering defects in materials and workmanship, including the imaging sensor, processor unit, and internal electronics. Exclusions typically include physical damage, misuse, and consumables. Extended warranties (up to 5 years) are available and highly recommended, often including priority support, predictive maintenance alerts, and loaner unit access during repairs. Distributors should confirm warranty transferability for resale or clinic relocation. |
| 5. How are firmware updates and software compatibility managed post-purchase, and are they included in the warranty or service plan? | In 2026, firmware and software updates are critical for maintaining compatibility with evolving imaging standards and cybersecurity protocols. Most manufacturers provide free over-the-air (OTA) firmware updates for the duration of the warranty. Advanced AI-driven image enhancement features and EHR integrations may require a subscription-based service plan. Ensure your procurement agreement specifies update frequency, cybersecurity compliance (e.g., GDPR, HIPAA), and long-term software support (LTS) commitments beyond the initial warranty period. |
Note: Specifications and support terms may vary by manufacturer. Always request a detailed technical compliance sheet and service level agreement (SLA) before purchase.
Need a Quote for Camera Optique Dentaire?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


