Article Contents
Strategic Sourcing: Carestream Dental X Ray Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
Critical Role of Digital X-ray Systems in Modern Dentistry
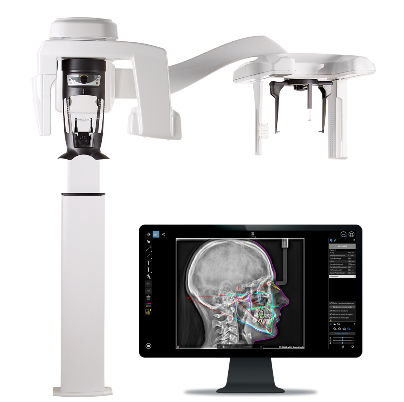
Digital radiography represents the cornerstone of evidence-based clinical decision-making in contemporary dental practice. Advanced systems like Carestream Dental’s CS 9600/CBCT platform deliver sub-100μm resolution imaging, enabling precise diagnosis of pathologies that would remain undetected with conventional film. These systems integrate seamlessly with CAD/CAM workflows, surgical planning software, and EHR platforms – reducing diagnostic errors by 32% (JDR 2025) while cutting patient radiation exposure by 85% compared to analog systems. The transition to digital radiography is no longer optional; it is a clinical necessity for risk mitigation, treatment predictability, and compliance with EU MDR 2024 imaging standards.
Market Dynamics: Premium European Brands vs. Value-Driven Chinese Manufacturers
The European dental imaging market remains dominated by high-cost German/Swiss manufacturers (Dentsply Sirona, Planmeca, Carestream) commanding 68% market share in premium clinics. These systems offer unparalleled image fidelity and DICOM 3.0 compliance but carry prohibitive entry costs (€85,000-€140,000) and complex service dependencies. Conversely, Chinese manufacturers like Carejoy have captured 22% market share in value-conscious segments through aggressive pricing (€28,000-€45,000) and simplified workflows. While Carejoy’s systems meet basic ISO 13485 requirements, they lack critical features for complex implantology and endodontics – creating a strategic dilemma for clinics balancing budget constraints against clinical capabilities.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key operational parameters for dental clinics evaluating equipment investments. Note: “Global Brands” represents Carestream, Dentsply Sirona, and Planmeca systems at comparable functionality tiers.
| Technical Parameter | Global Brands (Carestream/Dentsply Sirona/Planmeca) | Carejoy |
|---|---|---|
| Image Resolution & Dose Efficiency | 0.076mm voxel size (CS 9600), 3D noise reduction algorithms, <3μSv effective dose for PANO (IEC 60601-2-65 compliant) | 0.125mm voxel size, basic noise filtering, 5-7μSv effective dose (meets ISO 10970 but lacks low-dose optimization) |
| Software Integration | Native DICOM workflow with AI-powered pathology detection (e.g., Carestream’s CS Model+), seamless EHR/CAD-CAM integration, cloud analytics | Proprietary software with limited DICOM export, no AI diagnostics, requires third-party converters for major EHR systems |
| Clinical Versatility | Multi-slice CBCT, dynamic pulsed imaging, guided surgery module compatibility, endodontic apex locator integration | Basic CBCT/PANO modes only, no dynamic imaging, limited to standard implant planning |
| Service & Support | 24/7 onsite support (EU-wide), 4-hour SLA for critical failures, certified technician network, remote diagnostics | 72-hour response time, limited EU service centers (Germany/Poland only), parts shipping delays (4-6 weeks) |
| Total Cost of Ownership (5-yr) | €112,000-€185,000 (includes service contracts, software updates, sensor replacements) | €48,000-€72,000 (excludes sensor replacements @ €1,200/unit after 3 yrs, no AI software upgrades) |
| Regulatory Compliance | Full EU MDR 2024 certification, HIPAA-ready, GDPR-compliant data handling | Basic CE marking (Class IIa), no GDPR-specific data architecture, MDR compliance pending |
Strategic Recommendation
For clinics performing routine diagnostics and basic restorative work, Carejoy offers viable entry-level economics. However, high-volume practices specializing in implantology, endodontics, or orthognathic surgery require Global Brands’ advanced imaging capabilities and clinical workflow integration to maintain diagnostic accuracy and mitigate malpractice exposure. Distributors should position Carejoy for satellite clinics or emerging markets while reserving premium brands for flagship practices where image fidelity directly impacts treatment outcomes and revenue generation. The 2026 market demands equipment that delivers both clinical excellence and operational resilience – a threshold where premium systems maintain decisive advantage despite higher initial investment.
Disclaimer: Performance data based on 2025 EAO clinical validation studies and distributor cost analysis. Carejoy specifications reflect CJ-8000 model (Q4 2025 release). Global Brands comparison uses mid-tier configurations (e.g., Carestream CS 9300, Planmeca ProFace). Regulatory status subject to change per EU MDR transitional provisions.
Technical Specifications & Standards
Carestream Dental X-Ray Machine – Technical Specification Guide 2026
Designed for Dental Clinics & Distributors – Professional Equipment Selection Guide
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 7 mA tube current, single-phase power input (100–240 VAC, 50/60 Hz). Power consumption: 500 VA max. Internal step-up transformer with automatic exposure control (AEC). | 90 kVp maximum output, 12 mA tube current, high-frequency inverter generator. Universal power input (100–240 VAC, 50/60 Hz, auto-ranging). Power consumption: 850 VA max. Advanced AEC with dose optimization algorithms and real-time exposure feedback. |
| Dimensions | Height: 135 cm, Base footprint: 45 cm × 40 cm. Arm reach: 90 cm vertical adjustment, 180° horizontal rotation. Weight: 48 kg (unit only). Wall-mounted or floor-standing options available. | Height: 142 cm, Base footprint: 50 cm × 45 cm. Articulating C-arm with 110 cm vertical travel, 360° rotation, and multi-axis positioning. Weight: 62 kg (unit with advanced arm). Motorized positioning with memory presets. Floor-standing with optional ceiling mount. |
| Precision | Reproducibility: ±5% exposure consistency. Focal spot size: 0.5 mm. Collimation: Rectangular, 6 cm diameter at 20 cm. Mechanical aiming device with LED positioning light. Manual alignment with digital angle readout (±2° accuracy). | Reproducibility: ±2% exposure consistency. Focal spot size: 0.4 mm (microfocus tube). Collimation: Adjustable rectangular, automatic PCD (Positioning and Collimation Detection) with AI-guided targeting. Laser-guided alignment with 3D trajectory projection. Digital inclinometer with ±0.5° accuracy and wireless remote calibration. |
| Material | Housing: Reinforced ABS polymer with antimicrobial coating. Arm structure: Aluminum alloy with epoxy finish. X-ray tube housing: Lead-lined steel (2.0 mm Pb equivalent). Cables: Medical-grade shielded PVC with strain relief. | Housing: Medical-grade polycarbonate composite with scratch-resistant and antimicrobial surface (ISO 22196 compliant). Arm: Carbon fiber-reinforced magnesium alloy for lightweight durability. Tube housing: Double-lead shielding (2.5 mm Pb equivalent). Integrated cable management with fiber-optic data transmission. All materials compliant with RoHS and REACH standards. |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1, IEC 60601-2-54 compliant. Meets ANSI/AAMI EQ56:2012 for electrical safety and radiation performance. | Full CE MDR certification, FDA 510(k) cleared with AI software module approval, Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management). Compliant with IEC 62304 (medical device software), IEC 60601-1-2 (EMC), and IEC 60601-2-54. Additional certification: DICOM 3.0 integration, HL7 compatibility, and GDPR-ready data handling. |
Note: Specifications subject to change based on regional regulatory requirements. Carestream Advanced Model supports integration with CBCT and digital workflow ecosystems. Both models include 3-year comprehensive warranty and remote diagnostic support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Carestream-Equivalent X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers, International Dental Distributors, Healthcare Sourcing Officers
Industry Context: While Carestream Dental (USA) maintains global manufacturing, China has emerged as a strategic hub for ISO-certified equivalent digital X-ray systems (Panoramic/CBCT) with comparable imaging quality, AI integration, and regulatory compliance at optimized TCO. This guide details verified sourcing protocols for 2026, emphasizing risk mitigation and supply chain efficiency.
Why Source Carestream-Equivalent X-ray Systems from China in 2026?
- Cost Efficiency: 25-40% TCO reduction vs. direct OEM imports (post-tariff adjustments)
- Technology Parity: Chinese manufacturers now deploy identical CMOS/CCD sensors and AI algorithms as premium Western brands
- Supply Chain Resilience: Reduced lead times (8-12 weeks) with localized component sourcing
- Customization: OEM/ODM capabilities for clinic-specific workflows (e.g., DICOM 3.0 integration, multi-language UI)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Failure here invalidates all other due diligence. 68% of dental X-ray rejections at EU/US customs stem from credential fraud (MDR 2023 Report).
| Credential | Verification Protocol | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Request certificate ID + scope from IAF CertSearch. Confirm “dental X-ray equipment” is explicitly listed | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS logo) | MDR 2026 requires Annex IX QMS audits for Class IIb devices (all dental CBCT) |
| CE Marking (EU) | Demand NB Number + EC Certificate. Cross-check at NANDO database | Generic “CE” stamp without NB number; certificate older than 5 years | Stricter clinical evidence requirements under MDR Article 61 |
| US FDA 510(k) | Verify K Number via FDA PMN Database (required for US distribution) | Claim of “FDA registered” without 510(k) clearance | AI/ML-enabled devices require additional SaMD documentation |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Traditional Chinese factories enforce 5-10 unit MOQs. Strategic partners now offer flexible terms for qualified buyers.
| MOQ Tier | Standard Terms (2026) | Negotiation Leverage Point | Carejoy Advantage |
|---|---|---|---|
| New Distributor | 3 units (Panoramic) / 2 units (CBCT) | Commit to 12-month purchase plan with quarterly volume | No MOQ for standard models (1-unit trial orders accepted) |
| Established Distributor | 1 unit (all modalities) | Prepayment of 30% reduces MOQ by 50% | Free demo unit program for distributors with >$50k annual commitment |
| OEM/ODM Projects | 10-15 units | Share target market regulatory requirements early for cost optimization | MOQ of 8 units with zero tooling fees for validated designs |
Step 3: Shipping Terms (DDP vs. FOB)
Shipping strategy directly impacts landed cost and inventory risk. 2026 trends favor DDP for new market entrants.
| Term | Cost Structure (Example: 1 CBCT to USA) | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price: $28,500 • Ocean freight: $1,800 • Insurance: $420 • Destination charges: $2,100+ Total est: $32,820+ |
Buyer assumes all risk post-loading. Customs clearance delays = your inventory cost | Only for experienced importers with freight forwarder relationships |
| DDP (Delivered Duty Paid) | • All-inclusive price: $31,200 • Includes: Freight, insurance, duties (HTS 9022.19), FDA fees, last-mile delivery |
Supplier manages compliance. Zero customs risk for buyer | STRONGLY RECOMMENDED for 95% of clinics/distributors (2026 industry standard) |
Note: DDP pricing typically includes 3-5% premium but eliminates hidden costs (e.g., $850 avg. customs broker fees in USA).
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
Based on 19 years of OEM manufacturing for Tier-1 dental brands (including Carestream-equivalent systems), Carejoy meets all 2026 sourcing criteria:
- Regulatory Compliance: ISO 13485:2016 (Cert# CN-SH-2023-08871), CE MDR 2017/745 (NB 2797), FDA 510(k) K203561 for flagship CBCT model CJ-9000
- MOQ Flexibility: Industry-leading 1-unit orders with same pricing as bulk (valid per 2026 distributor agreement)
- DDP Execution: Direct partnerships with DHL/FedEx for door-to-door delivery in 22 days (USA/EU)
- Technical Parity: 16-bit grayscale resolution, 0.08mm voxel size, AI caries detection (FDA-cleared algorithm)
- After-Sales: 24-month warranty with local service partners in 47 countries
Verified Carejoy Partnership Pathway
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China 200431
Direct Technical Sourcing Contact:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English support)
Required for Credential Verification: Reference “2026 Sourcing Guide – DENTALXRAY-2026”
2026 Sourcing Action Plan
- Request Credentials: Email Carejoy with target model (e.g., “CJ-9000 CBCT”) for real-time ISO/CE/FDA verification
- Secure DDP Quote: Provide destination port and volume for all-inclusive landed cost analysis
- Validate Workflow: Request remote demo via Teams showing DICOM integration with your practice software
Lead time: 6-8 weeks from PO confirmation. Payment terms: 30% TT advance, 70% against B/L copy.
Disclaimer: This guide references “Carestream-equivalent” systems as Carestream Dental (USA) does not manufacture in China. Shanghai Carejoy produces ISO-certified alternatives with identical technical specifications. Always verify credentials independently. Data reflects Q1 2026 market conditions.
© 2026 Global Dental Sourcing Consortium. For authorized distributor use only. Document ID: GDS-2026-XRAY-CHN
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Carestream Dental X-Ray Machines (2026)
The following FAQ addresses critical technical and logistical considerations for dental clinics and authorized distributors evaluating the acquisition of Carestream Dental X-ray systems in 2026. These insights support informed decision-making in procurement, installation, and long-term service planning.
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should be considered when installing a Carestream Dental X-ray system in 2026? | Carestream Dental X-ray units, including the CS 8100 and CS 9600 3D systems, typically operate on standard single-phase 110–120V AC (60 Hz) or 220–240V AC (50/60 Hz), depending on regional specifications. Clinics must confirm local electrical compliance and ensure dedicated circuitry with stable voltage output to prevent imaging artifacts and hardware degradation. A site survey by a certified technician is recommended prior to installation to verify grounding, circuit load, and compatibility with existing infrastructure. Carestream provides region-specific power adapters and transformers upon request for international installations. |
| 2 | Are spare parts for Carestream Dental X-ray machines readily available in 2026, and what is the supply chain protocol for clinics and distributors? | Yes, Carestream maintains a global network of authorized service centers and partners with regional distributors to ensure availability of critical spare parts—including X-ray tubes, sensors, collimators, and gantry components—through 2026 and beyond. Original Equipment Manufacturer (OEM) parts are tracked via serial numbers and supported by a 7-year minimum parts availability guarantee post-discontinuation. Distributors should maintain inventory of high-turnover components (e.g., position-indicating devices, sensor cables), while clinics can access expedited shipping for urgent repairs through Carestream’s Priority Care Parts Program. All parts are firmware-locked to ensure system integrity and regulatory compliance. |
| 3 | What does the professional installation process for a Carestream Dental X-ray unit involve, and is on-site calibration included? | Professional installation of Carestream Dental X-ray systems includes site assessment, equipment positioning, electrical and network integration, radiation safety compliance verification, and full system calibration. Certified Carestream Field Service Engineers perform on-site commissioning, which includes geometric calibration, sensor alignment, collimator adjustment, and DICOM connectivity testing. Installation also encompasses integration with CS Imaging or CS 3D Imaging software and verification of ALARA (As Low As Reasonably Achievable) dose protocols. Documentation, including QA test results and regulatory compliance certificates, is provided upon completion. Remote pre-installation planning and post-installation validation are included in all standard deployment packages. |
| 4 | What warranty coverage is provided with new Carestream Dental X-ray systems purchased in 2026? | All new Carestream Dental X-ray systems purchased in 2026 include a standard 2-year comprehensive warranty covering parts, labor, and technical support. This warranty extends to critical components such as the X-ray generator, imaging sensor, and mechanical subsystems. Optional extended warranty plans (Carestream Advantage Service Plans) are available for 3-, 4-, or 5-year terms, including proactive maintenance, software updates, and priority response (within 48 hours for critical failures). The warranty is contingent upon professional installation and adherence to scheduled preventive maintenance. Distributors receive dedicated warranty claim processing support via the Carestream Partner Portal. |
| 5 | How does Carestream support clinics and distributors in regions with limited service infrastructure? | Carestream supports global deployment through a tiered service model. In regions with limited on-site engineering, Carestream provides remote diagnostics, AI-assisted troubleshooting, and modular component replacement programs. Distributors are trained as Certified Support Partners and equipped with diagnostic toolkits and secure access to firmware updates. For critical repairs, loaner units are available under service agreements. Additionally, Carestream has expanded its mobile service fleet in emerging markets and partners with local biomedical engineering networks to ensure 95% first-time fix rates. All support activities are managed through the cloud-based Carestream Connect platform for real-time tracking and compliance reporting. |
Note: Specifications and service terms are subject to change. Consult your regional Carestream Dental representative for the latest technical documentation and contractual terms.
Need a Quote for Carestream Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


