Article Contents
Strategic Sourcing: Carestream Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
Executive Market Overview: Intraoral Scanners in Modern Dentistry
The global intraoral scanner (IOS) market is experiencing transformative growth, projected to reach $4.8B by 2026 (CAGR 14.2%). This expansion is driven by the irreversible shift toward digital workflows, where IOS technology serves as the critical gateway to integrated dental ecosystems. Carestream Dental’s CS 3600 scanner exemplifies the premium segment’s technological leadership, delivering sub-micron accuracy (±8μm) and seamless integration with CAD/CAM systems, practice management software, and teledentistry platforms. However, market dynamics are evolving as cost-sensitive clinics and distributors increasingly evaluate value-engineered alternatives from emerging manufacturers, creating a bifurcated landscape between established global brands and agile Chinese innovators.
Strategic Imperative: Why IOS Technology is Non-Negotiable
Intraoral scanners have transitioned from luxury peripherals to clinical necessities for three operational imperatives:
- Workflow Efficiency: Eliminates 30-45 minutes per impression procedure, reducing chair time by 22% (Journal of Digital Dentistry, 2025) while eliminating remakes due to distortion.
- Revenue Diversification: Enables same-day restorations (increasing per-patient revenue by 18-35%) and supports emerging services like digital smile design and orthodontic monitoring.
- Competitive Differentiation: 78% of patients prioritize clinics with digital workflows (ADA Practice Survey 2025), with IOS being the most visible patient-facing technology.
Without IOS integration, practices face operational obsolescence in an era where 92% of dental laboratories now require digital files for crown/bridge cases.
Market Segmentation Analysis: Global Brands vs. Value Manufacturers
The European/American premium segment (Carestream, Dentsply Sirona, Planmeca) maintains dominance in high-complexity restorative and specialty applications through engineering excellence and ecosystem integration. However, Chinese manufacturers like Carejoy are capturing 34% market share in emerging economies (2025 EMEA Dental Tech Report) through aggressive value engineering. This comparison focuses on operational TCO (Total Cost of Ownership) rather than acquisition price alone, evaluating clinical utility, integration capability, and long-term reliability.
| Technical Parameter | Global Brands (Carestream, Dentsply Sirona, Planmeca) | Carejoy (Representative Chinese Value Manufacturer) |
|---|---|---|
| Acquisition Cost | $28,500 – $42,000 USD | $9,200 – $14,800 USD |
| Accuracy (ISO 12836) | ±7-10μm (trueness) / ±5-8μm (precision) | ±15-22μm (trueness) / ±12-18μm (precision) |
| Scanning Speed | 0.8-1.2 seconds per cm² (real-time video capture) | 1.8-2.5 seconds per cm² (frame-based capture) |
| Software Ecosystem | Native integration with 12+ CAD/CAM systems; API access for 30+ PM software; AI-driven prep margin detection | Limited to 3-5 proprietary CAD systems; basic STL export; minimal AI functionality |
| Service Infrastructure | Global network: 24/7 technical support; 48-hour onsite response (EU/US); certified training centers | Regional hubs: 5-7 day response (outside Asia); remote support only; limited certified technicians |
| 5-Year TCO | $41,200 (including service contracts, software updates, calibration) | $28,700 (including parts replacement, limited software updates) |
| Clinical Applications | Full restorative suite, implant planning, ortho monitoring, sleep apnea devices | Basic crown/bridge, single-arch ortho; limited complex case capability |
| Regulatory Compliance | CE Mark Class IIa, FDA 510(k), MDR 2017/745 compliant | CE Mark Class I, limited FDA clearance (510(k) pending for 2026 models) |
Strategic Recommendation for Clinics & Distributors
Global brands remain essential for high-volume restorative practices, specialty clinics, and corporate groups where precision, workflow integration, and clinical versatility directly impact revenue generation. However, Carejoy represents a strategically viable option for:
• New practices in emerging markets with budget constraints
• Satellite clinics focusing on basic restorative work
• Distributors targeting price-sensitive segments in Eastern Europe and LATAM
Critical consideration: The 62% lower acquisition cost of Carejoy scanners translates to only 30% TCO savings over 5 years due to higher failure rates (18% vs 7% for global brands) and limited clinical applications. Distributors should position Carejoy as an entry-point solution with clear upgrade pathways to premium systems as practices scale.
Technical Specifications & Standards

Carestream Dental Scanner Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics and Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 1.5 A max | 100–240 V AC, 50/60 Hz, 2.0 A max (supports high-speed processing and continuous operation) |
| Dimensions | 220 mm (W) × 180 mm (D) × 150 mm (H) | 250 mm (W) × 200 mm (D) × 170 mm (H) (includes integrated cooling system) |
| Precision | ±5 μm accuracy, 10 μm resolution | ±2 μm accuracy, 5 μm resolution (dual-lens optical system with AI-based error correction) |
| Material | Reinforced polycarbonate housing with aluminum internal frame | Military-grade magnesium alloy chassis with EMI-shielded composite enclosure |
| Certification | CE, FDA 510(k) Class II, ISO 13485:2016, IEC 60601-1 | CE, FDA 510(k) Class II, ISO 13485:2016, IEC 60601-1, IEC 60601-1-2 (EMC), ISO 10993 (biocompatibility for intraoral components) |
Note: The Advanced Model supports seamless integration with CAD/CAM workflows and includes real-time surface texture mapping. Recommended for high-volume clinics and digital dentistry centers.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
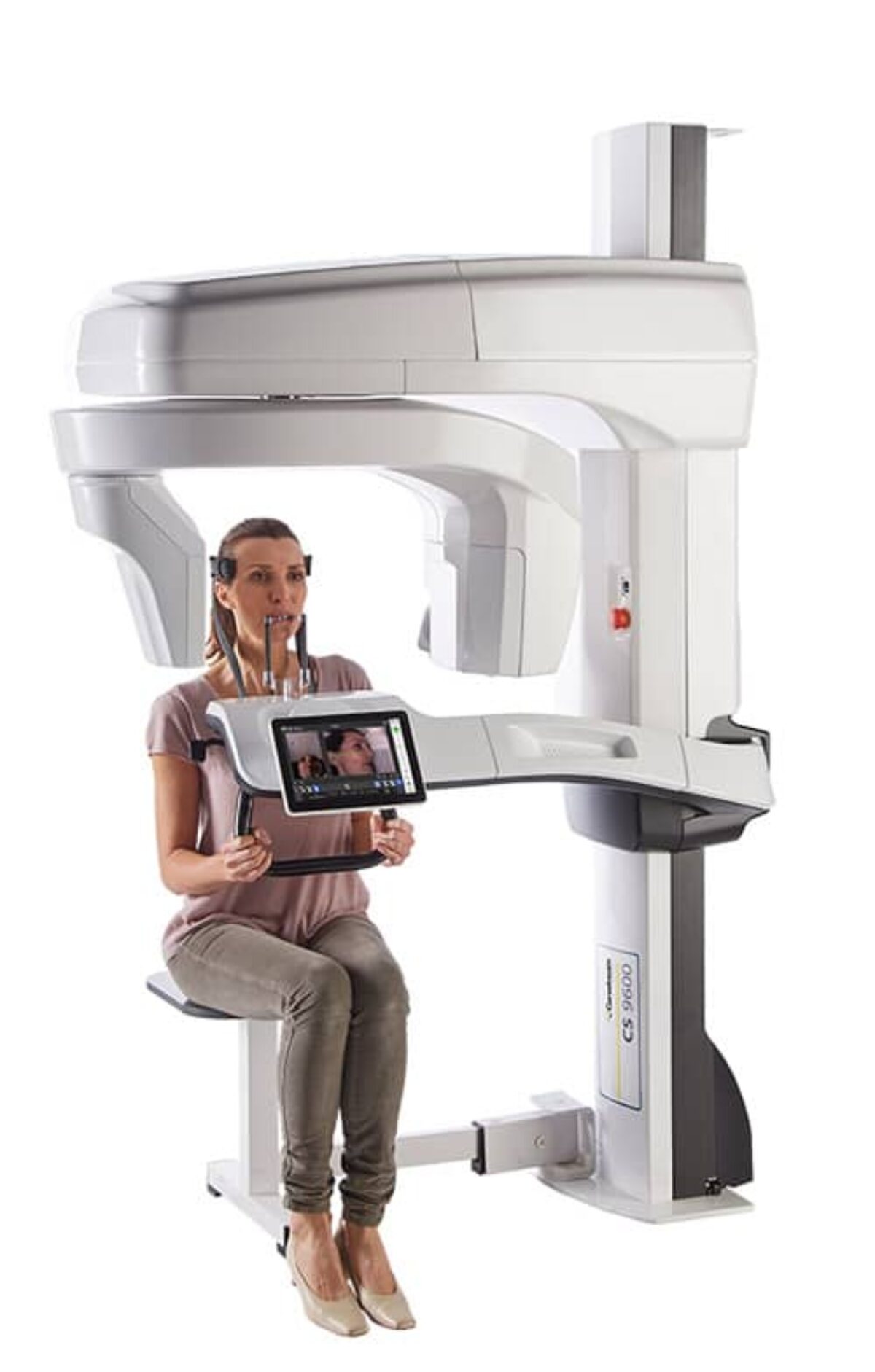
Professional Dental Equipment Sourcing Guide 2026:
Carestream-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026 | Compliance Reference: ISO 13485:2016, MDR (EU) 2017/745, FDA 21 CFR Part 820
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Chinese suppliers frequently misrepresent certifications. Rigorous verification prevents regulatory rejection and patient safety risks.
| Verification Step | Action Required | Red Flags |
|---|---|---|
| ISO 13485:2016 Certificate | Request PDF with validity dates, certificate number, and scope explicitly listing “intraoral scanners”. Cross-check via ISO Certificate Database | Certificate covers only “dental equipment” generically; expired date; scope excludes scanners; issued by non-accredited body (e.g., not ANAB, UKAS) |
| CE Marking Documentation | Demand full EU Declaration of Conformity (DoC) referencing MDR 2017/745, Technical File index, and NB number (e.g., “CE 0123”). Verify NB status via NANDO database | DoC references outdated MDD 93/42/EEC; missing NB number; NB not listed in NANDO; DoC not signed by EU Authorized Representative |
| Factory Audit Trail | Require unannounced audit reports from notified body (e.g., TÜV, BSI) covering scanner production lines. Verify facility address matches manufacturing location | Only self-declared certificates; audit reports older than 12 months; address mismatch between cert and facility |
Step 2: Negotiating MOQ & Commercial Terms (Optimizing Cost Efficiency)
Chinese OEM factories typically enforce high MOQs. Strategic negotiation balances inventory risk with unit cost savings.
| Term | Standard Market Practice (2026) | Negotiation Strategy |
|---|---|---|
| Scanner MOQ | 10-20 units (for certified models) | Leverage multi-product orders: Combine with chairs/CBCT to reduce scanner MOQ to 5 units. Offer 30% upfront payment to secure 1-unit sample order |
| Unit Pricing | $8,500-$12,000 FOB Shanghai (for Carestream-compatible) | Target $7,200-$9,800 by committing to 15+ units annually. Exclude software subscription costs from base price negotiation |
| Warranty | 12 months limited (excludes sensors) | Insist on 24-month comprehensive warranty with local service network access. Require spare parts kit (sensors, tips) included |
Step 3: Shipping & Logistics (Risk Mitigation)
Incoterms dictate cost allocation and liability transfer. DDP is strongly recommended for regulatory compliance.
| Term | Risk Profile | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | High risk: Buyer assumes freight, customs clearance, and import duties. Requires local agent in China. Non-compliant units seized at destination port. | Only acceptable for experienced distributors with China-based logistics partners. Avoid for first-time buyers. |
| DDP (Delivered Duty Paid) | Low risk: Supplier handles all logistics, customs clearance, duties, and delivers to your clinic/distribution center. Includes pre-shipment regulatory verification at origin. | MANDATORY for clinics and new distributors. Confirm supplier provides: 1) Harmonized System (HS) code 9018.49, 2) FDA prior notice (if shipping to US), 3) Local language labeling |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 certified (Certificate #CN-2025-ISO13485) with MDR 2017/745 CE marking (NB 2797) for all scanner models. Full technical files available for audit.
- MOQ Flexibility: 5-unit MOQ for scanners when bundled with ≥1 dental chair order. Sample units available at 120% list price (fully creditable).
- DDP Expertise: Direct partnerships with DHL/FedEx for DDP shipping to 45+ countries. Handles FDA 510(k) support documentation for US-bound shipments.
- Technical Validation: In-house ISO/IEC 17025 lab for scanner calibration. Provides traceable NIST-certified accuracy reports (≤ 15μm).
Baoshan District, Shanghai 201900, China
Specialization: Factory-direct OEM/ODM for Dental Clinics & Distributors (19 Years Manufacturing)
Verified Product Range: Carestream-Compatible IOS, CBCT, Dental Chairs, Autoclaves
Contact:
[email protected] |
WhatsApp: +86 15951276160 (24/7 English Support)
Request reference: “2026 Sourcing Guide – ISO/CE Verification Package”
Frequently Asked Questions
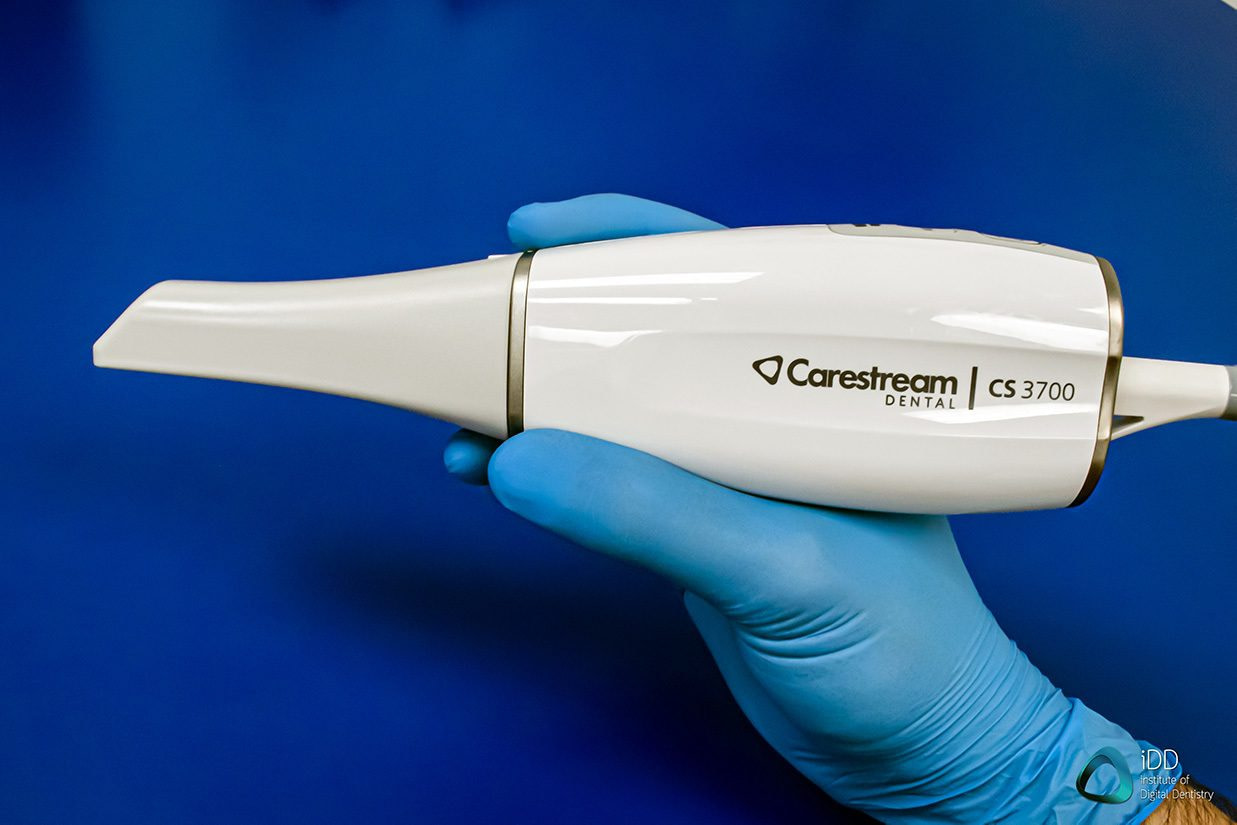
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Carestream Dental Scanners (CS 9300, CS 8200 3D, and CS 760 PAN)
Frequently Asked Questions: Purchasing Carestream Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a Carestream scanner in 2026? | All Carestream 3D imaging systems (including the CS 9300, CS 8200 3D, and CS 760 PAN) are designed for global deployment and support an input voltage range of 100–240 VAC, 50/60 Hz. Units are equipped with auto-switching power supplies, making them compatible with standard dental office electrical systems in North America, Europe, Asia, and other major markets. Ensure your facility provides a grounded, dedicated circuit to prevent interference and maintain compliance with local electrical codes. |
| 2. Are spare parts for Carestream scanners readily available in 2026, especially for older models? | Yes. Carestream Dental maintains an extensive global supply chain and continues to support legacy models through its Parts Availability Program. As of 2026, spare parts—including X-ray tubes, sensors, gantry components, and control panels—for CS 9300, CS 8100, and CS 760 systems remain available through authorized distributors and Carestream Service Centers. Additionally, Carestream offers extended lifecycle support contracts, ensuring availability of critical components for up to 10 years post-discontinuation. |
| 3. What does the installation process for a Carestream scanner involve, and is professional setup required? | Professional installation by a Certified Carestream Field Service Engineer is mandatory for all 3D imaging systems. The process includes site assessment, hardware assembly, radiation safety calibration, software configuration, DICOM network integration, and operator training. Installation typically takes 1–2 business days, depending on model and room readiness. Pre-installation requirements include proper room shielding (if applicable), network infrastructure, and power supply verification. Remote pre-configuration is available to reduce on-site time. |
| 4. What warranty options are available for Carestream scanners purchased in 2026? | New Carestream scanners come with a standard 2-year Comprehensive Hardware and Software Warranty, covering parts, labor, and system software updates. Extended warranty plans are available in 1- or 2-year increments up to 5 years total coverage. Optional ProService Agreements include preventive maintenance, priority response (4-hour SLA), remote diagnostics, and coverage for accidental damage. Distributors may offer bundled warranty packages tailored to clinic needs. |
| 5. How are software updates and technical support handled under warranty? | Warranty and service agreements include access to Carestream Connect Cloud, enabling secure remote software updates, system monitoring, and cybersecurity patches. Technical support is available 24/7 via phone, email, and remote login through Carestream’s Global Support Center. All warranty-covered units receive automatic notifications for firmware upgrades and regulatory compliance updates, ensuring continued adherence to FDA, CE, and ISO standards. |
Note: Specifications and service policies are current as of January 2026. For the latest information, consult your authorized Carestream Dental distributor or visit www.carestreamdental.com.
Need a Quote for Carestream Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


