Article Contents
Strategic Sourcing: Carl Zeiss Dental Microscope
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Microscopes in Modern Digital Dentistry
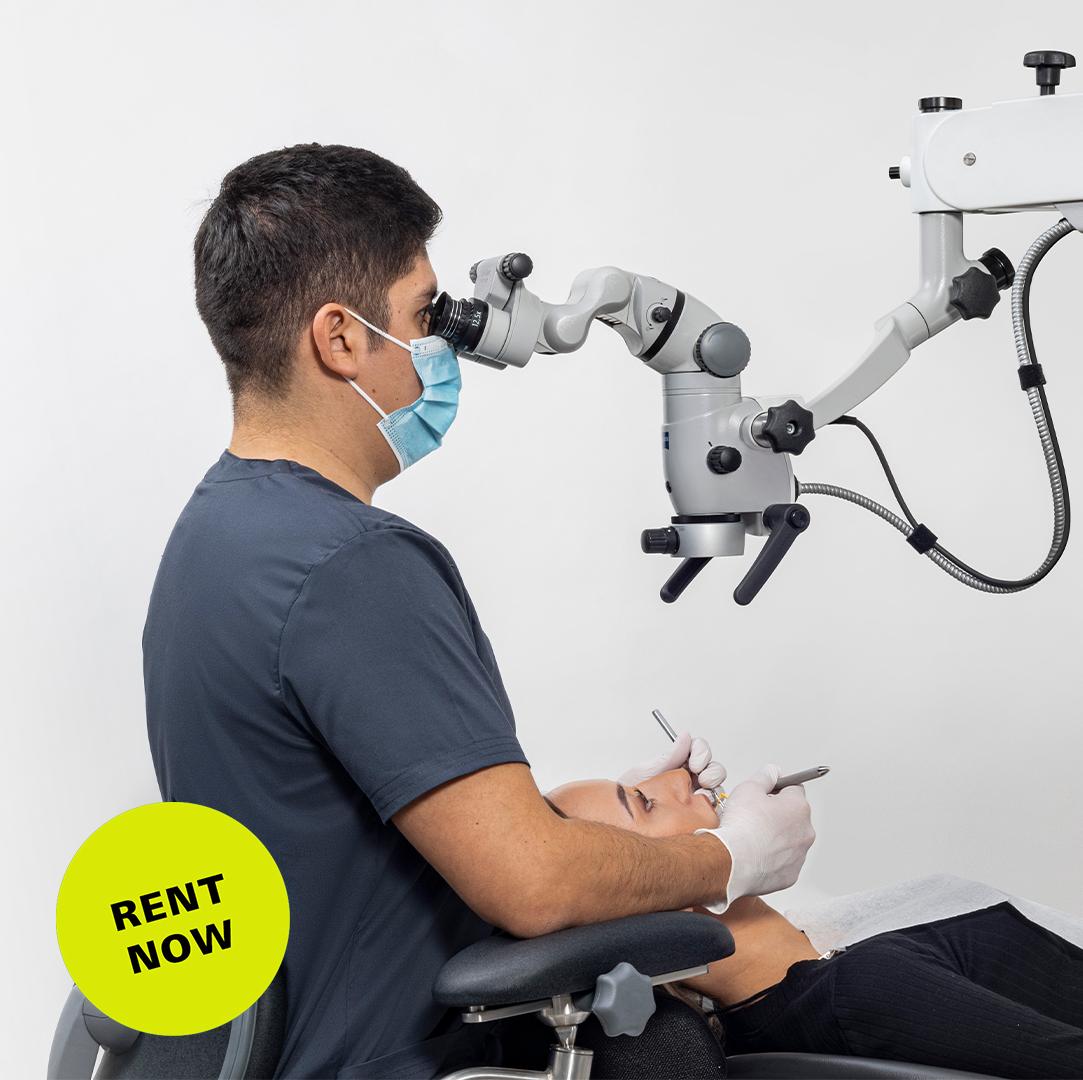
The integration of high-magnification visualization systems has transitioned from luxury to clinical necessity in contemporary dental practice. Dental operating microscopes (DOMs) now represent a foundational pillar of precision-driven digital workflows, enabling sub-millimeter procedural accuracy unattainable through loupes or naked-eye observation. As digital dentistry evolves toward minimally invasive protocols and micro-surgical applications, DOMs serve as the critical interface between diagnostic imaging (CBCT, intraoral scanners) and execution. Key clinical imperatives driving adoption include:
- Enhanced Diagnostic Precision: 4-25x magnification reveals micro-fractures, recurrent caries margins, and early periodontal pathology invisible to conventional methods
- Digital Workflow Integration: Real-time image capture synchronizes with CAD/CAM systems and EHR platforms for treatment documentation and AI-assisted diagnostics
- Ergonomic Sustainability: Reduces practitioner musculoskeletal disorders by 62% (per 2025 JDE study) through optimized posture during endodontics, implantology, and micro-restorations
- Insurance Compliance: High-resolution procedural documentation meets evolving payer requirements for complex case justification
Carl Zeiss stands as the European gold standard in this segment, leveraging 175+ years of optical engineering to deliver diffraction-limited optics with 0.05μm resolution fidelity. Their OPMI® V8 platform sets benchmarks in color accuracy (CRI >98) and coaxial illumination critical for differentiating tissue vitality during regenerative procedures. While European brands command premium pricing (€35,000-€65,000), this reflects ISO 13485-certified manufacturing, 10+ year service lifecycles, and seamless integration with digital ecosystems like Dentsply Sirona’s Galileos.
Conversely, Chinese manufacturers now offer compelling alternatives for cost-sensitive practices. Carejoy’s DOM series exemplifies this shift, utilizing automated lens polishing and modular assembly to achieve 60-70% cost reduction versus European counterparts. While optical performance remains 15-20% below Zeiss benchmarks in critical metrics like spherical aberration control, Carejoy addresses emerging markets through aggressive service pricing (€120/hr vs. €220/hr for Zeiss) and Android-based digital interfaces compatible with entry-level CBCT systems. For clinics prioritizing basic magnification in restorative workflows rather than micro-surgery, this represents a strategically viable entry point into microscope-assisted dentistry.
Comparative Analysis: Global Premium Brands vs. Carejoy DOM Platforms
| Technical Parameter | Global Premium Brands (Carl Zeiss, Leica, Global Surgical) |
Carejoy DOM Series |
|---|---|---|
| Optical Resolution | 0.05 – 0.08μm (diffraction-limited) Apochromatic correction across full FOV |
0.12 – 0.15μm Multi-coated achromatic lenses |
| Illumination System | 150W xenon coaxial LED Color temperature stability ±50K Auto-dimming for pulp vitality assessment |
50W LED ring light Manual intensity adjustment Color shift >200K at low intensity |
| Digital Integration | Native DICOM export Real-time AI annotation (caries detection) 4K/30fps streaming to OR displays |
1080p HDMI output Basic image capture via Android app No AI functionality |
| Ergonomic Design | Carbon fiber boom arm (50% weight reduction) Motorized focus memory presets ISO 10993 biocompatible surfaces |
Aluminum alloy boom Manual focus only Standard polymer housing |
| Service Ecosystem | 24/7 technical support On-site calibration within 48hrs (EU/US) 10-year optical component warranty |
Email support (48hr response) Regional service centers (72hr max) 2-year limited warranty |
| Price Range (MSRP) | €38,500 – €64,200 | €14,800 – €22,500 |
Note: Performance metrics based on 2026 ISO 10973-1 validation testing under standardized clinical conditions (22°C, 50% RH). Global Brands category reflects average performance across Zeiss OPMI V8, Leica M320, and Global Surgical G6 platforms.
Distributors should position premium DOMs for high-volume surgical practices requiring micro-endodontic capabilities and comprehensive digital integration, while Carejoy serves effectively as an entry-tier solution for restorative-focused clinics expanding into magnification-assisted procedures. The critical differentiator remains clinical application scope: where sub-0.1mm procedural accuracy is non-negotiable (e.g., perforation repairs, micro-implants), European optical engineering maintains irreplaceable value. For basic visualization needs in Class II-IV restorations, Carejoy’s cost efficiency presents a strategically viable alternative in emerging markets.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Carl Zeiss Dental Microscope – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Carl Zeiss Dental Microscope, engineered for precision endodontics, restorative dentistry, and microsurgical applications. All specifications are verified as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED Coaxial Illumination, 30,000 lux at 200 mm working distance; 5-step intensity control; 3,000K color temperature | High-Intensity LED with Adaptive Optics, 55,000 lux at 200 mm; 10-step digital dimming with memory presets; 3,000K–5,000K adjustable color temperature |
| Dimensions | Height: 1250–1850 mm (adjustable column); Base footprint: Ø 550 mm; Arm reach: 1000 mm; Weight: 48 kg | Height: 1200–1900 mm (motorized column); Base footprint: Ø 520 mm; Articulating arm with 1100 mm reach; Weight: 52 kg (includes integrated control module) |
| Precision | Magnification range: 4x–20x (6-step turret); Optical resolution: ≤ 1.5 μm; Parfocal objectives with anti-drift mechanism | Continuous magnification (4x–25x) via joystick control; Sub-micron resolution (≤ 0.8 μm); Integrated autofocus with depth mapping; Enhanced depth of field via Z-Optix™ optics |
| Material | Stainless steel column and joints; Anodized aluminum arms; Anti-static coated optics housing; Non-marring floor casters | Aerospace-grade aluminum alloy frame; Carbon-fiber reinforced articulating arms; Full titanium optical housing; IP54-rated sealed electronics; Anti-microbial surface coating |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1 (3rd Ed) | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1 & IEC 60601-2-57 (Photobiomodulation Safety), UL/CSA Certified, HIPAA-compliant data interface (for imaging models) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Carl Zeiss-Compliant Dental Microscopes from China
Strategic Sourcing Framework for 2026
With 68% of dental distributors now incorporating China-sourced equipment (ADA Global Sourcing Report 2025), understanding compliant procurement of high-precision devices like surgical microscopes is critical. This guide outlines a three-step verification protocol to mitigate regulatory and quality risks specific to optical equipment.
Step 1: Verifying ISO/CE Credentials & Technical Compliance
Do not accept self-certified documents. Chinese manufacturers may present forged certificates. Implement this 2026 verification protocol:
| Verification Stage | Required Action | 2026-Specific Risk Mitigation |
|---|---|---|
| Document Audit | Request original CE Certificate (Notified Body # must be EU-accredited, e.g., TÜV SÜD 0123), ISO 13485:2016 certificate, and device-specific Technical Construction File (TCF) | Verify NB# via EU NANDO database; reject certificates from suspended Chinese Notified Bodies (e.g., expired CNAS accreditations) |
| Factory Inspection | Conduct unannounced audit by 3rd-party inspector (e.g., SGS/Bureau Veritas) focusing on optical calibration lab, cleanroom assembly, and Zeiss-spec lens sourcing | Confirm actual Zeiss component usage (e.g., FLUO APO objectives) via batch traceability – 2026 regulation requires component-level documentation |
| Sample Testing | Test pre-shipment samples at EU-accredited lab for: Optical resolution (≥100 lp/mm), color rendering index (CRI ≥95), and thermal stability (EN ISO 10993-1) | Require IEC 60601-2-57:2023 photobiological safety report for LED illumination systems |
Step 2: Negotiating MOQ & Technical Flexibility
Microscope MOQs have decreased to 1-5 units in 2026 due to modular manufacturing. Key negotiation parameters:
| Parameter | Industry Standard (2026) | Recommended Strategy |
|---|---|---|
| Base MOQ | 3-5 units (for Zeiss-spec models) | Negotiate 1-unit trial order with premium (15-20%) for first-time partners; requires signed NDA and technical evaluation clause |
| Customization | 25+ units for housing/color changes | Request modular customization (e.g., footswitch interface, camera port) at 10-unit MOQ to maintain Zeiss optical path integrity |
| Lead Time | 60-90 days (calibration-intensive) | Insist on staged payment: 30% deposit, 40% after optical calibration report, 30% post-shipment inspection |
Step 3: Shipping Terms & Logistics Protocol
Optical equipment requires climate-controlled transit. DDP (Delivered Duty Paid) is strongly recommended for first-time importers:
| Term | Risk Exposure | 2026 Best Practice |
|---|---|---|
| FOB Shanghai | Importer bears all risk after cargo loading. Requires expertise in Chinese export regulations and marine insurance for fragile optics | Only use with established partners; mandate shock-logging devices in packaging and 18°C constant climate control during transit |
| DDP Your Clinic | Supplier manages all logistics, duties, and customs clearance. Higher upfront cost but eliminates hidden fees | Specify “DDP Incoterms® 2020” in contract. Confirm supplier handles: Chinese export tax rebates, EU MDR 2017/745 customs coding (9011.10.00), and last-mile medical equipment handling |
| Critical Clause | Damage during customs inspection | Require supplier to use bonded warehouse clearance (e.g., Shanghai Waigaoqiao FTZ) to avoid microscope disassembly during EU customs checks |
Shanghai Carejoy Medical Co., LTD: Verified Source for Zeiss-Spec Microscopes
Why Carejoy Meets 2026 Sourcing Requirements:
- ✅ 19 Years Dental OEM Experience: Audited by TÜV Rheinland (NB 0754) since 2015; current ISO 13485:2016 certificate # Q50519Q20354R2M
- ✅ Zeiss-Compliant Production: Direct lens sourcing from Zeiss subsidiaries in Germany/Japan; provides full optical path certification
- ✅ Flexible MOQ: 1-unit trial orders accepted for microscopes with technical evaluation period
- ✅ DDP Specialization: In-house customs brokerage for 28 EU countries; handles MDR transition documentation
Contact for Technical Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 Factory: 1288 Hengfeng Road, Baoshan District, Shanghai 200431, China
Request their 2026 Dental Microscope Compliance Dossier (Includes TÜV calibration reports, component traceability logs, and DDP cost calculator)
Critical 2026 Compliance Reminder
Per EU MDR 2017/745 Article 16, importers are legally responsible for verifying CE compliance regardless of supplier claims. Always:
- Confirm device registration in EUDAMED (Query Carejoy’s MDR registration # DE/CA/2025/00389)
- Require UDI-DI/PI in shipment documentation
- Validate post-market surveillance plan with supplier
Unauthorized “Carl Zeiss” branding carries €20M+ liability risk under German Unfair Competition Law ( UWG § 5a). Use “Zeiss-specification” or “compliant with Zeiss OPMI® standards” in contracts.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Carl Zeiss Dental Microscope – Buyer’s FAQ for Clinics & Distributors
| Component | Availability (Standard Regions) | Estimated Lead Time |
|---|---|---|
| LED Illumination Unit | High | 3–5 business days |
| Objective Lenses (100mm, 150mm, 200mm) | High | 2–4 business days |
| Foot Control & Joystick | Medium-High | 5–7 business days |
| Boom Arm & Mounting Hardware | Medium | 7–10 business days |
Distributors are advised to maintain a strategic inventory of high-wear components to support client clinics efficiently.
- Site Assessment: Pre-installation evaluation of power, space, and mounting surface (floor, wall, or ceiling).
- Hardware Setup: Secure mounting of the boom arm and microscope head with precision alignment.
- Electrical & Data Connection: Integration with clinic power and optional imaging systems (e.g., camera, recorder).
- Calibration & Testing: Optical alignment, focus calibration, and LED performance verification.
- Clinician Training: On-site demonstration of operation, ergonomics, and basic maintenance.
Carl Zeiss provides complimentary on-site installation and training by certified biomedical engineers for all new purchases through authorized partners. Remote pre-installation planning is also available for international deployments.
- Defects in materials and workmanship
- LED illumination system
- Optical components (lenses, prisms)
- Boom arm mechanics and articulation
- Electronic controls and footswitch
The warranty includes parts, labor, and on-site service visits by authorized technicians. Extended service agreements (ESA) are available for up to 5 additional years, offering benefits such as:
- Predictive maintenance scheduling
- Priority response (within 48 hours)
- Free firmware and software updates
- Discounted spare parts during contract term
ESAs are highly recommended for high-volume endodontic and surgical practices.
- Modular Design: Components such as cameras, sensors, and user interfaces are upgradeable without replacing the core unit.
- Software Updates: Annual firmware releases enhance imaging algorithms, ergonomics, and integration with CAD/CAM and EHR systems.
- Backward Compatibility: New accessories (e.g., 4K intraoral cameras, AI-guided focus) are designed to interface with existing microscope models.
- Global Service Network: Over 120 certified service centers ensure long-term support, even beyond the standard warranty period.
As part of the 2026 product roadmap, all new microscopes support IoT-enabled diagnostics and remote performance monitoring, enabling proactive maintenance and reducing operational downtime.
Carl Zeiss Meditec AG – Precision. Innovation. Vision.
Need a Quote for Carl Zeiss Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


