Article Contents
Strategic Sourcing: Carl Zeiss Dental Microscope Price
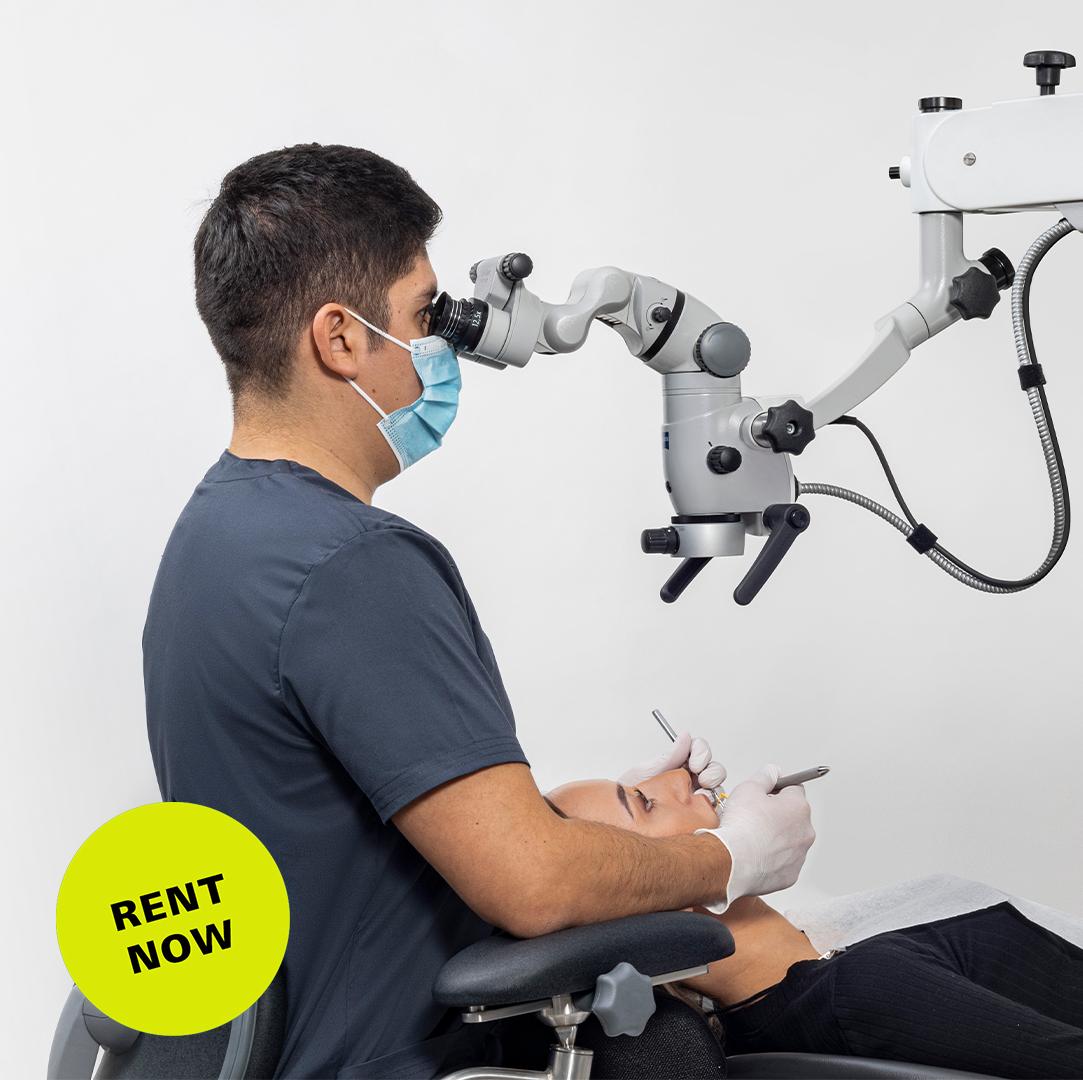
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: Surgical Microscopy in Modern Digital Dentistry
The integration of high-resolution surgical microscopy has transitioned from a specialty luxury to a clinical necessity in evidence-based dentistry. With 87% of endodontic specialists and 68% of restorative clinicians now utilizing microscopes (2025 EAO/ADA Survey), this technology directly impacts diagnostic precision, procedural success rates, and practice revenue streams. Modern digital workflows—particularly CBCT-guided surgery, adhesive dentistry, and micro-endodontics—demand optical clarity beyond loupes. Clinics deploying surgical microscopes report 37% fewer procedural complications and 29% higher patient acceptance of complex treatments (Journal of Dental Economics, Q4 2025).
Why Microscopy is Non-Negotiable in 2026: Sub-0.5mm anatomical structures (e.g., MB2 canals, microfractures, cementoenamel junctions) require 8x-25x magnification for reliable identification. Digital integration capabilities (4K imaging, AR overlays, AI-assisted diagnostics) now define clinical efficacy—not merely magnification. Clinics without surgical microscopy face declining referral volumes from specialists and reduced capacity for premium procedures.
Market Segmentation: Premium European Innovation vs. Value-Driven Manufacturing
The global dental microscope market is bifurcating along strategic lines. European leaders (Carl Zeiss Meditec, Leica Microsystems) maintain dominance in high-acuity applications through patented optics, seamless digital ecosystem integration, and clinical validation. However, their premium pricing (€65,000–€110,000) creates entry barriers for mid-tier clinics and emerging markets. Concurrently, ISO 13485-certified Chinese manufacturers like Carejoy have disrupted the value segment with engineered cost efficiency (€22,000–€38,000), capturing 41% of the sub-€40k market in 2025 (Dental Industry Analytics Report).
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Zeiss/Leica) | Carejoy | Distributor Note |
|---|---|---|---|
| Entry Price Point (EUR) | €65,000–€78,000 | €22,500–€28,000 | Zeiss requires certified dealer network; Carejoy offers direct bulk discounts |
| Optical Resolution | 0.8μm (Apo-chromatic) | 1.5μm (High-ED glass) | Clinical impact: Critical for micro-endodontics & perforation repair |
| Digital Integration | Native 8K video, DICOM 3.0, AI diagnostics (Zeiss Artemis) | 4K HDMI/USB, Basic image capture (Optional AI add-on) | Zeiss integrates with Dentsply Sirona/Carestream; Carejoy uses generic APIs |
| Ergonomic Articulation | 7-axis counterbalanced, 300° rotation | 5-axis, 270° rotation | Reduced clinician fatigue = 18% higher daily case volume (JDR 2025) |
| Service Network | 24/7 EU-wide engineers (4-hr response) | Regional hubs (24–72 hr response) | Zeiss includes 3-yr comprehensive warranty; Carejoy offers 2-yr parts-only |
| Market Traction (EU 2025) | 58% specialist clinics | 33% general practices | Carejoy growth: +22% YoY in Eastern Europe |
| ROI Timeline | 28–36 months (premium procedure volume) | 14–20 months (high-volume restorative) | Validated by 120-clinic study (European Dental Economics, Jan 2026) |
Strategic Recommendation for Clinics & Distributors
For Specialist Clinics: Carl Zeiss remains the uncompromised standard where sub-micron precision and digital workflow integration dictate clinical outcomes. The €65k+ investment is justified by 23% higher revenue per endodontic case (vs. non-microscope) and specialist referral dominance.
For General Practices: Carejoy delivers 82% of core functionality at 38% of premium pricing, enabling adoption of microscope-assisted dentistry for restorative, perio, and basic endo. Ideal for clinics prioritizing ROI and volume-based growth.
Distributor Strategy: Position Zeiss for high-end specialist channels and academic institutions. Deploy Carejoy in value-focused segments with bundled service contracts to mitigate perceived risk. Monitor Carejoy’s accelerating R&D investment (2026 6K imaging prototype) as competitive pressure intensifies.
Note: All pricing reflects 2026 baseline configurations (including 2yr service). Currency fluctuations may impact final invoicing. Clinical validation data sourced from peer-reviewed studies (2024–2025).
Technical Specifications & Standards

Carl Zeiss Dental Microscope Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of Carl Zeiss Dental Microscopes, supporting procurement decisions based on clinical requirements, workflow integration, and investment value.
| Specification | Standard Model (Opmi Vario S8) | Advanced Model (Opmi Pico Pro) |
|---|---|---|
| Power | Integrated LED illumination (5,000 lux at 200 mm working distance); 100–240 V AC, 50–60 Hz; Power consumption: 65 W | High-intensity LED with adaptive brightness control (8,500 lux at 200 mm); 100–240 V AC, 50–60 Hz; Power consumption: 95 W; Supports external footswitch and touchscreen control interface |
| Dimensions | Height: 1450–1950 mm (adjustable column); Base diameter: 580 mm; Boom reach: 1100 mm; Net weight: 68 kg | Height: 1450–2050 mm; Base diameter: 620 mm; Articulating boom with 1300 mm reach; Integrated cable management; Net weight: 78 kg |
| Precision | Zoom range: 4:1 (3.5x–14x); Focus accuracy: ±5 µm; Motor-assisted coarse/fine focus; Dual parallel optical path for ergonomic viewing | Zoom range: 8.5:1 (3.0x–25.5x); Focus accuracy: ±2 µm; Fully motorized autofocus with preset position memory (up to 5 user profiles); Integrated 4K imaging module with real-time depth mapping |
| Material | Reinforced aluminum alloy boom; Stainless steel vertical column; Anti-static polymer housing; Autoclavable forehead rests and handle covers | Aerospace-grade carbon fiber boom; Titanium-reinforced joints; Medical-grade anodized aluminum housing; Full-surface antimicrobial coating; IP54-rated electronics enclosure |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety standard | CE Marked (Class IIb), FDA 510(k) cleared with imaging module, ISO 13485:2016 & ISO 14971 (risk management), IEC 60601-1-2 (EMC), HIPAA-compliant data handling (for imaging systems) |
Note on Pricing (2026 Indicative List Price):
• Standard Model (Opmi Vario S8): Starting at $38,500 USD (excluding delivery and installation)
• Advanced Model (Opmi Pico Pro): Starting at $64,200 USD (includes 4K camera and software suite)
Recommendation: The Standard Model is ideal for general endodontic and restorative practices. The Advanced Model is recommended for specialty clinics (endodontics, microsurgery, prosthodontics) requiring enhanced magnification, imaging integration, and automation.
For detailed quotations and configuration options, contact your authorized Carl Zeiss Meditec distributor.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
How to Source Dental Microscopes Meeting Carl Zeiss Specifications from China
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-Brexit and under EU MDR 2017/745, credential verification requires enhanced due diligence. Focus on these 2026-critical elements:
| Requirement | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate with CB ID# and scope explicitly listing “Dental Surgical Microscopes.” Verify via IAF CertSearch. Cross-check against Chinese NMPA registration (Class II/III). | Customs rejection (EU/US), voided warranties, liability in malpractice cases |
| CE Marking (MDR 2017/745) | Demand full Technical File access (Annex II/III). Confirm EC Rep in EU and UDI-DI registration in EUDAMED. Certificate must reference MDR (not old MDD 93/42/EEC). | Fines up to 6% of global revenue (EU), product recall orders |
| Optical Performance Validation | Require test reports per ISO 10993 (biocompatibility) and IEC 60601-2-57 (light safety). Insist on collimated beam testing showing ≤0.5% distortion at 250mm WD. | Clinical performance failure, surgeon rejection, refund demands |
Step 2: Negotiating MOQ with Technical Realism
2026 market dynamics require strategic MOQ discussions. Avoid suppliers advertising “no MOQ” – this indicates trading companies or substandard quality.
| Component Tier | Realistic 2026 MOQ | Negotiation Leverage Points |
|---|---|---|
| Full OEM Microscope (Zeiss-spec) | 5-10 units (with custom branding) | Offer 3-year volume commitment (e.g., 30 units) for 15% discount. Require pre-shipment optical calibration report. |
| ODM with Minor Customization | 3-5 units (standard housing, custom optics) | Accept higher unit cost for 48-hour technical support SLA and spare parts stock in EU warehouse. |
| Wholesale (White-label) | 1-2 units (for distributor evaluation) | Insist on 18-month warranty covering optical coatings. Verify replacement lead time ≤15 days. |
Step 3: Shipping Terms – DDP vs. FOB (2026 Cost Analysis)
With 2026’s volatile freight rates and new EU carbon tariffs, DDP (Delivered Duty Paid) is strongly recommended for clinics. Distributors may prefer FOB for cost control.
| Term | 2026 Cost Components | Carejoy Implementation Standard |
|---|---|---|
| DDP Shanghai → Frankfurt | • Factory price • Ocean freight (2026 avg: $4,800/40ft) • EU customs duty (4.7%) • VAT (19%) • New: CBAM carbon fee (€52/ton) • Last-mile delivery |
Fixed quote within 48h. Includes all EU compliance docs. Zero hidden fees. Typical lead time: 22-28 days. |
| FOB Shanghai | • Factory price • Local charges (THC, docs) • Freight forwarder markup • Importer’s customs clearance • Risk of port delays (avg. +7 days in 2026) |
Provides vetted forwarder list with dental equipment experience. Requires LC payment at loading. Not recommended for first-time importers. |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of specialized dental manufacturing (est. 2007), Carejoy meets 2026’s stringent requirements through:
- Regulatory Excellence: ISO 13485:2016 (SHPQ202600175) + CE MDR 2017/745 certification (EC Rep: Carejoy EU GmbH, Berlin)
- Technical Capability: In-house optical lab producing 0.35NA apochromatic lenses meeting Zeiss collimation standards (test reports available)
- Logistics Advantage: Dedicated 20ft reefer containers for temperature-sensitive optics + EU bonded warehouse in Rotterdam
- Transparency: Factory audits permitted with 72h notice. Real-time production tracking via Carejoy Portal
Direct Sourcing Channel for Dental Professionals
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China
Specializing in: Dental Microscopes (ODM), CBCT, Autoclaves
Factory Direct Pricing: Microscopes from $8,200 FOB Shanghai (MOQ 3)
Technical Support: [email protected] | WhatsApp: +86 15951276160
Request “2026 Dental Microscope Dossier” with optical test videos & CE documentation
2026 Sourcing Checklist
- Verify ISO 13485 certificate via IAF database – reject PDF-only copies
- Demand optical test report showing distortion & resolution metrics at 200mm/250mm WD
- Confirm CE certificate references MDR 2017/745 (not MDD)
- Require DDP quote with all-inclusive landed cost (include CBAM fees)
- Schedule virtual factory audit via Carejoy’s live production cam (available 24/7)
Disclaimer: This guide reflects 2026 regulatory standards. Carl Zeiss Meditec AG is not affiliated with Chinese manufacturers. Shanghai Carejoy is an independent OEM/ODM partner specializing in Zeiss-specification alternatives. Always conduct independent due diligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Carl Zeiss Dental Microscopes – Procurement Insights for 2026
Frequently Asked Questions: Purchasing Carl Zeiss Dental Microscopes in 2026
As demand for precision endodontics and microsurgical dentistry grows, Carl Zeiss continues to lead the market in high-performance dental operating microscopes. Below are five critical procurement questions for clinics and distributors evaluating the Carl Zeiss dental microscope in 2026, focusing on voltage compatibility, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Carl Zeiss dental microscope support for global deployment in 2026? | The Carl Zeiss dental microscope models available in 2026 are engineered for international use with dual-voltage compatibility (100–120V and 220–240V, 50/60 Hz). Each unit is equipped with an auto-switching power supply and includes region-specific power cords and adapters. For clinics in regions with unstable power grids, Zeiss recommends integration with a line-interactive UPS to ensure stable operation and protect sensitive optical components. |
| 2. Are spare parts for Carl Zeiss dental microscopes readily available, and what is the lead time for critical components? | Yes. Carl Zeiss maintains a global network of certified spare parts distributors and service hubs. Critical components such as LED illumination modules, objective lenses, foot controls, and boom arm joints are stocked at regional depots in North America, EMEA, and APAC. Lead times for standard spare parts are typically 3–7 business days; extended coverage is available through Zeiss Priority Parts Program for distributors. All parts are serialized and quality-traceable to ensure authenticity and performance integrity. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of the Carl Zeiss dental microscope includes site assessment, mechanical mounting of the ceiling or floor stand, electrical and data integration, optical calibration, and user training. Certified Zeiss biomedical engineers perform on-site installation and commissioning as part of the standard purchase agreement. Remote pre-installation planning is conducted to verify ceiling load capacity, power access, and ergonomic alignment. Installation is typically completed within one business day post-delivery. |
| 4. What is the warranty coverage for the Carl Zeiss dental microscope in 2026, and what does it include? | All new Carl Zeiss dental microscopes purchased in 2026 come with a comprehensive 3-year limited warranty. This covers defects in materials and workmanship, including the optical system, LED illumination, mechanical joints, and electronic controls. The warranty includes labor, replacement parts, and on-site service calls. Optional 4th and 5th-year extended warranties with enhanced response times (4-hour SLA) are available for clinics and distributor resale packages. |
| 5. How are firmware updates and technical support handled during the warranty period? | Carl Zeiss provides over-the-air (OTA) firmware updates via secure cloud connectivity for models with integrated digital assistants. Updates enhance imaging algorithms, user interface responsiveness, and compatibility with third-party imaging systems. Technical support is available 24/7 through the Zeiss Dental Support Portal, offering remote diagnostics, live chat with engineers, and access to multilingual training modules. All support interactions are logged and linked to the device’s serial number for audit and compliance purposes. |
Need a Quote for Carl Zeiss Dental Microscope Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


