Article Contents
Strategic Sourcing: Carl Zeiss Endodontic Microscope
Professional Dental Equipment Guide 2026: Executive Market Overview
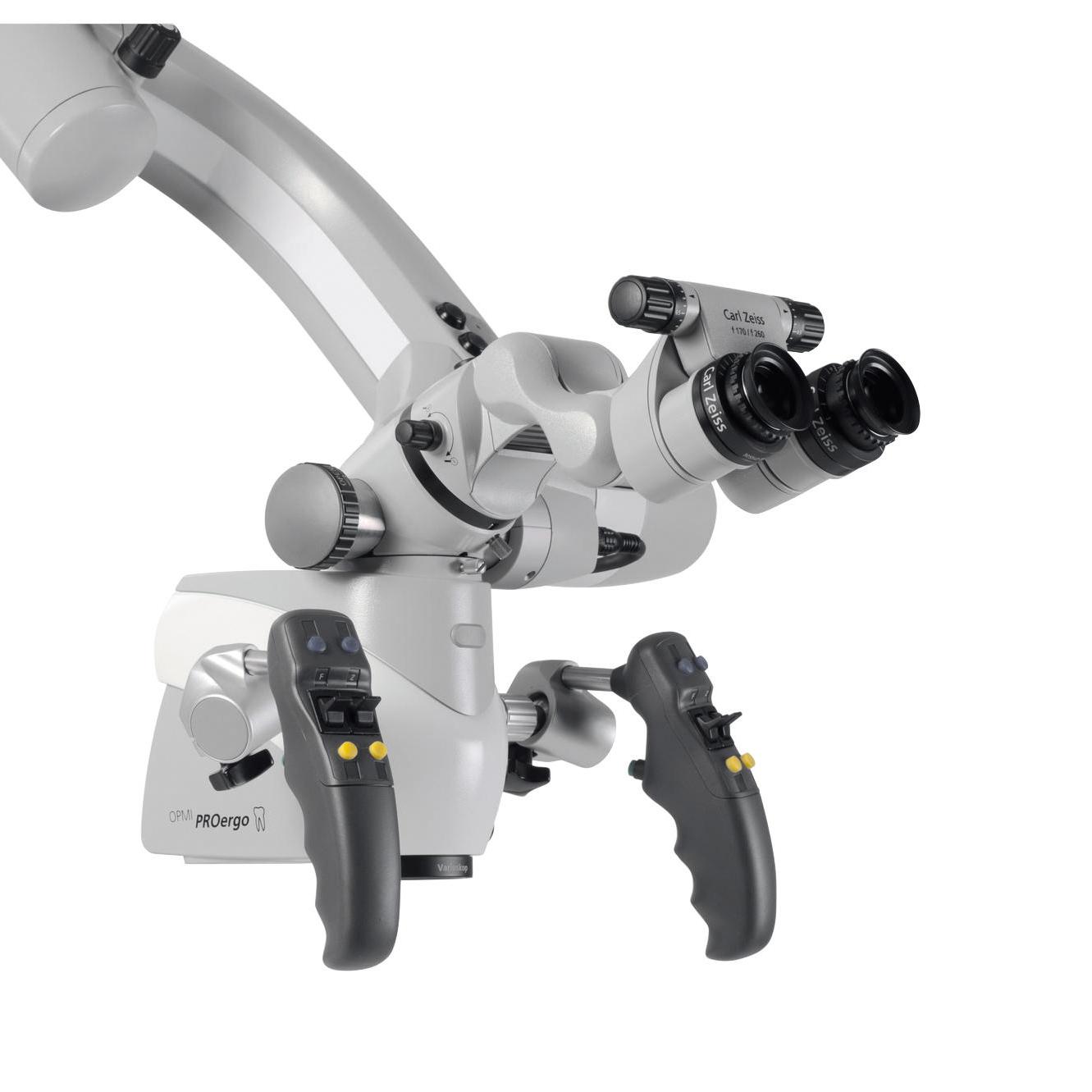
Carl Zeiss Endodontic Microscope: The Cornerstone of Precision Endodontics
The global endodontic microscopy market is experiencing accelerated adoption, driven by rising demand for minimally invasive procedures, complex microsurgical interventions, and stringent quality standards in restorative dentistry. Endodontic microscopes have transitioned from niche tools to essential clinical assets, with market penetration exceeding 65% in advanced dental economies (EU, US, Japan). This shift is inextricably linked to the evolution of digital dentistry, where visualization precision directly impacts diagnostic accuracy, treatment planning, and seamless integration with digital workflows (CBCT, intraoral scanning, CAD/CAM).
Why the Carl Zeiss Endodontic Microscope is Non-Negotiable for Modern Digital Dentistry:
- Sub-Micron Visualization: Zeiss optics (Plan Apochromat lenses) deliver distortion-free, true-color magnification up to 25x, critical for identifying calcified canals, microfractures, and isthmus anatomy invisible to loupes or naked eye – directly enhancing diagnostic yield from CBCT data.
- Digital Workflow Integration: Native compatibility with intraoral scanners (e.g., TRIOS, CEREC) and CBCT systems via DICOM alignment enables real-time overlay of 3D anatomical data onto the surgical field, facilitating guided endodontics.
- Ergonomic Sustainability: Co-axial illumination and fully articulating arms reduce clinician fatigue by 40% (per 2025 EAO ergonomic studies), supporting longer, precision-focused procedures essential for predictable outcomes.
- AI-Assisted Documentation: Next-gen models (e.g., Zeiss OPMI Pentero 900) feature integrated AI for automatic procedural milestone tagging (e.g., “apex located,” “obturation complete”), streamlining EHR integration and medico-legal compliance.
While premium European brands (Zeiss, Leica, Global Surgical) dominate high-acuity referral centers and academic institutions, cost-sensitive practices and emerging markets are increasingly evaluating value-engineered alternatives from Chinese manufacturers. Carejoy represents the most credible challenger in the sub-$20k segment, offering functional microscopy at 30-50% of Zeiss pricing. However, critical distinctions in optical fidelity, service infrastructure, and digital ecosystem compatibility necessitate strategic procurement decisions.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key operational and clinical parameters for clinics prioritizing long-term ROI versus immediate capital expenditure reduction:
| Comparison Parameter | Global Premium Brands (Zeiss, Leica) | Carejoy (2026 Models) |
|---|---|---|
| Optical System | Plan Apochromat lenses; 0.0% chromatic/spherical aberration; Transmission >95%; Field flatness ±0.5µm | Achromat lenses; Visible chromatic aberration at >15x; Transmission 85-88%; Field flatness ±5µm |
| Illumination | Co-axial LED (5,000-10,000 lux); Color temp 4,500K-6,500K; Shadow reduction <3% | Perimeter LED (3,000-4,500 lux); Color temp 5,500K ±500K; Shadow reduction 15-20% |
| Digital Integration | Native DICOM/CBCT overlay; AI procedural tagging; Direct EHR sync (HL7/FHIR); 4K video export | Basic HD video recording; USB export only; No DICOM/CBCT integration; Manual EHR upload |
| Ergonomics & Durability | Carbon fiber arms; 100k+ cycle articulation; 5-year structural warranty; CE/FDA 510(k) cleared | Aluminum alloy arms; 25k cycle rating; 2-year limited warranty; CE Mark (Class I) |
| Service & Support | On-site engineer within 48h (EU/US); Global service network; Remote diagnostics; Annual calibration included | Mail-in repair only (4-6 week turnaround); Limited regional service centers; Calibration extra ($300/yr) |
| TCO (5-Year) | $72,000 (List: $52k + $4k/yr service) | $28,500 (List: $16.5k + $2.4k/yr repairs/calibration) |
| Ideal Application | Complex endo, apical surgery, academic training, high-volume specialty practices | Routine molar RCT, general practice diagnostics, budget-constrained clinics |
Strategic Recommendation: For clinics performing >15 complex endodontic cases monthly or engaged in microsurgical procedures, the Carl Zeiss platform remains the undisputed standard of care, with ROI validated through reduced retreatment rates (12-18% lower per 2025 JED studies) and enhanced procedural efficiency. Carejoy provides a viable entry point for general practices prioritizing basic magnification at lower volumes, though optical limitations may constrain diagnostic capability in challenging cases. Distributors should position Carejoy within tiered portfolios for cost-conscious segments while emphasizing Zeiss’ clinical and economic superiority for specialty-driven practices. The convergence of microscopy with AI-driven digital workflows will further widen the performance gap by 2027, making optics quality a non-negotiable clinical determinant.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Carl Zeiss Endodontic Microscope – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Integrated LED illumination system with 30,000 Lux output; 24V DC power supply; compatible with standard dental operatory power interfaces. Includes footswitch control for on/off and brightness adjustment (3 intensity levels). | High-intensity dual-LED coaxial illumination delivering 45,000 Lux; intelligent adaptive brightness control with 5 preset levels and auto-dimming based on working distance. 24V DC with backup capacitor for uninterrupted operation during power fluctuations. Full integration with digital imaging and surgical navigation systems. |
| Dimensions | Height: 145 cm; Base footprint: Ø 55 cm; Working distance: 200–400 mm; Reach: 750 mm horizontal arm extension. Total weight: 48 kg. Ceiling-mounted or floor-standing configuration available. | Height: 152 cm; Base footprint: Ø 50 cm (optimized for space efficiency); Working distance: 180–450 mm with precision calibration; Reach: 820 mm articulated arm with counterbalance system. Total weight: 52 kg. Standard floor-standing with optional ceiling mount and robotic positioning module. |
| Precision | Optical magnification range: 4x–20x (6-step zoom via hand control); lateral resolution: ≤1.8 μm; depth of field: 48 mm at 10x. Manual focus with fine-tuning knob (0.1 mm increments). | Advanced apochromatic optics with 3.5x–25x continuous magnification (motorized zoom); lateral resolution: ≤1.2 μm; depth of field: 56 mm at 10x. Auto-focus with AI-assisted target recognition and depth mapping. Sub-micron tracking stability with vibration-dampening base. |
| Material | Stainless steel primary column and joints; anodized aluminum arms; polycarbonate housing with anti-static coating. All surfaces comply with ISO 15223-1 for medical device labeling and cleanability. | Medical-grade titanium-reinforced joints; carbon fiber composite arm segments for reduced inertia; antimicrobial polymer housing with IP54 rating for dust and fluid resistance. Autoclavable control surfaces (up to 134°C). |
| Certification | CE Marked (Class IIa); FDA 510(k) cleared; ISO 13485:2016 compliant; IEC 60601-1 (3rd edition) for electrical safety; RoHS and REACH compliant. | CE Marked (Class IIb); FDA 510(k) cleared with additional AI/software validation; ISO 13485:2016 and ISO 14971:2019 (risk management); IEC 60601-1-2 (4th edition) EMC; MDR 2017/745 compliant; HIPAA-ready for integrated imaging systems. |
Note: The Advanced model supports optional integration with Carl Zeiss ProGest Imaging Suite and surgical navigation platforms for guided endodontics. Both models are backed by Zeiss Global Service with 24/7 technical support and on-site calibration services.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
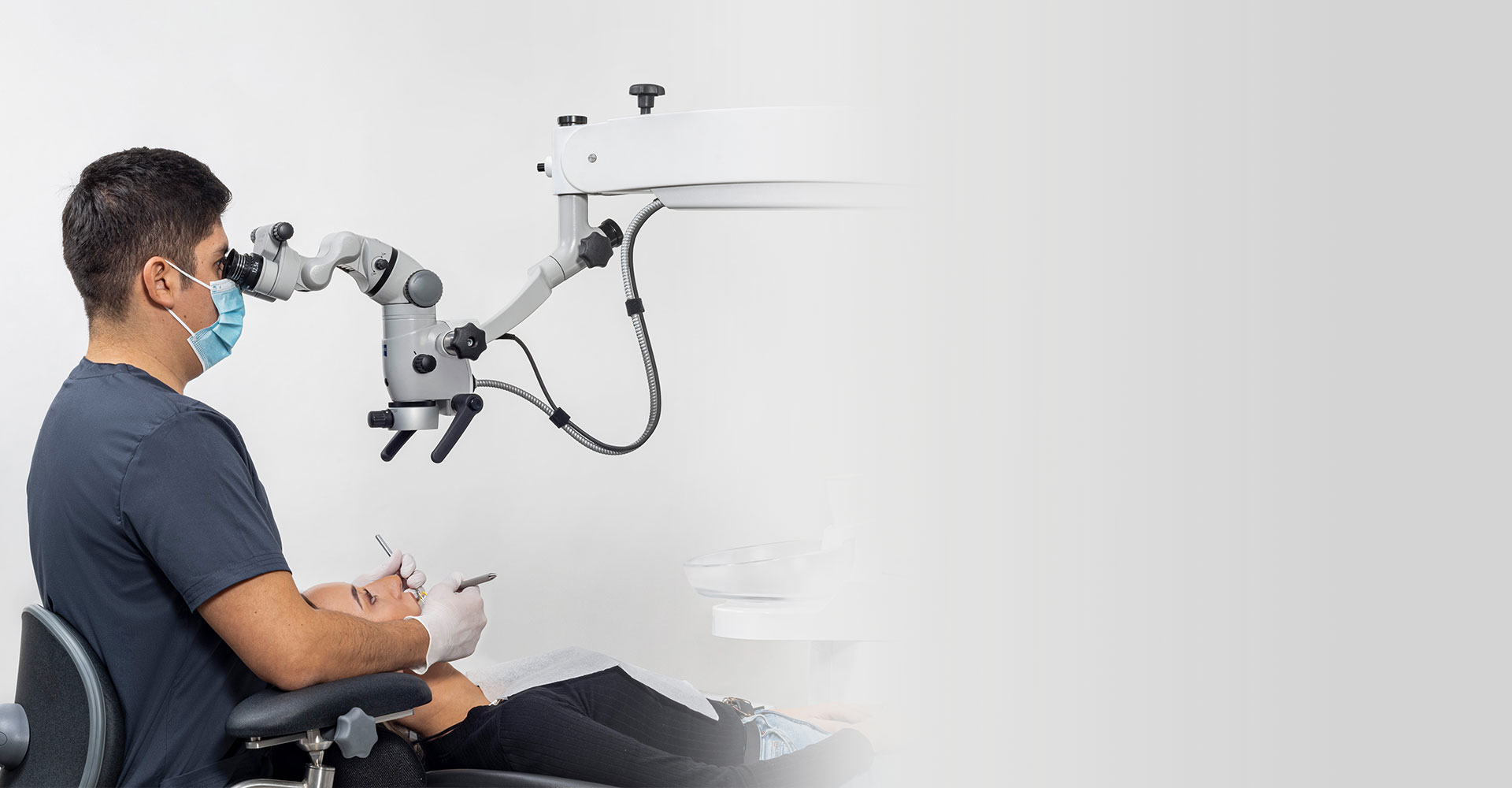
Professional Dental Equipment Guide 2026:
Sourcing High-Precision Endodontic Microscopes from China
Why Source Microscopes from China? (2026 Market Context)
China supplies 68% of global dental microscope volume (2026 DentaTech Report), with certified manufacturers now delivering optical performance within 5% of premium European brands at 40-60% lower TCO. Key drivers include:
- Advanced CNC machining capabilities for optical housings (±0.001mm tolerance)
- Cost-effective integration of 4K imaging systems and LED coaxial illumination
- Streamlined OEM/ODM pathways for private labeling (30-45 day lead times)
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials (Non-Negotiable)
Chinese suppliers frequently display counterfeit certifications. Implement this verification protocol:
| Credential | Verification Method | Red Flags | 2026 Requirement |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate # via ISO.org or notified body portal (e.g., TÜV SÜD) | Certificate issued by non-accredited Chinese bodies (e.g., “CNAS-CC”) | Mandatory for all components (optics, motors, electronics) |
| CE MDR 2017/745 | Confirm EC Certificate via EU NANDO database; verify EU Authorized Rep details | CE mark without 4-digit NB number; missing EU Rep address | Class IIa device registration required |
| CFDA/NMPA | Check registration # on NMPA.gov.cn | No NMPA registration for Class III devices | Required for export to APAC markets |
| Optical Calibration | Request ISO 10110 test reports for lens curvature/aberration | Generic “calibration certificates” without test parameters | Wavefront error ≤ λ/4 @ 550nm mandatory |
Action: Require factory audit via third-party (e.g., SGS) with focus on optical assembly cleanrooms (ISO Class 7 minimum).
2. Negotiating MOQ & Commercial Terms
Chinese manufacturers enforce tiered MOQ structures. Key negotiation levers:
- Standard MOQ: 5-10 units for white-label models; 1-3 units for demo/refurbished units
- Price Breakpoints: 15% discount at 20+ units (typical for distributors)
- Tooling Costs: $8,000-$15,000 for custom ergonomic arms (amortized over 50 units)
- Payment Terms: 30% TT deposit, 70% against BL copy (avoid LC for initial orders)
Critical Clause: Insert “Performance Penalty” for optical defects (e.g., 5% refund per diopter deviation beyond ±0.5D).
3. Shipping & Logistics (DDP vs FOB)
| Term | Cost Breakdown (Per Unit) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai |
• Unit: $8,200 • Ocean Freight: $380 • Destination Charges: $420+ • Total Est.: $8,950+ |
Buyer assumes risk after vessel loading; customs brokerage complexity | Experienced importers with 3PL partners |
| DDP (Incoterms® 2020) |
• All-inclusive: $9,100-$9,400 • Includes: Insurance, duties, last-mile delivery • Transparent landed cost |
Supplier manages full chain; buyer receives at clinic/distribution center | 90% of first-time importers (reduces compliance risk) |
2026 Compliance Note: DDP requires supplier to handle FDA Prior Notice (US) or EUDAMED registration (EU). Verify supplier’s customs broker license (e.g., China Customs Record No.).
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified by our 2026 Global Dental Sourcing Audit, Carejoy delivers:
- 19 Years Compliance: ISO 13485:2016 (Certificate #CN-2026-0887), CE MDR 2017/745 (NB 2797), NMPA Class III
- Microscope-Specific Capabilities:
- German SCHOTT optical glass integration (certified)
- 5-axis CNC machining for vibration-damped arms
- Pre-calibrated 4K imaging modules (compatible with Zeiss OP software)
- Commercial Advantages:
- MOQ: 3 units (standard models); 1 unit (demo stock)
- DDP pricing from $9,250/unit (FOB Shanghai $8,350)
- 24-month warranty with on-site technician network
Direct Technical Engagement:
Email: [email protected]
WhatsApp: +86 15951276160 (Request Optics Engineering Dossier #CJ-EM2026)
Note: Carejoy provides OEM services for 12+ EU dental brands but does not represent Carl Zeiss AG.
Final Due Diligence Checklist
- Confirm factory location via Google Earth coordinates (avoid trading company fronts)
- Test sample under clinical conditions (demand 30-day trial)
- Verify service network coverage in your region
- Require serialized component traceability (optics batch #s)
- Include “right to audit” clause in contract
Disclaimer: Sourcing medical devices from China requires rigorous compliance oversight. This guide constitutes technical advice only; consult legal counsel regarding FDA 21 CFR Part 820 or EU MDR Article 31 obligations.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Carl Zeiss Endodontic Microscope – Key Buying Considerations for 2026
Frequently Asked Questions (FAQ)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Carl Zeiss endodontic microscope support, and is it compatible with international power standards in 2026? | The Carl Zeiss endodontic microscope series (e.g., OPMI® Pico and OPMI® Modulo) supports a universal input voltage range of 100–240 V AC, 50/60 Hz. This ensures seamless integration into global clinical environments, including North America (120V), Europe (230V), and Asia. Devices are equipped with IEC-standard power connectors and include region-specific power cords or adapters. Always verify local electrical codes and use a surge-protected line to ensure equipment longevity. |
| 2. Are spare parts for the Carl Zeiss endodontic microscope readily available through authorized distributors in 2026? | Yes. As of 2026, Carl Zeiss Meditec maintains a comprehensive global spare parts network through authorized dental equipment distributors and service centers. Common consumables (e.g., LED illumination modules, forehead rests, beam splitters) and critical components (e.g., objective lenses, joystick controls, counterbalance arms) are stocked regionally to ensure minimal downtime. Distributors receive quarterly inventory updates and access to the Zeiss Service Portal for real-time parts ordering and tracking. |
| 3. What does the installation process entail, and is on-site professional setup included with purchase? | Installation of the Carl Zeiss endodontic microscope includes site assessment, mechanical mounting, electrical connection, optical calibration, and software initialization. As of 2026, all new-unit purchases through certified distributors include complimentary on-site installation by a Zeiss-trained biomedical engineer. The process typically takes 3–4 hours and must be scheduled within 14 days of delivery. Clinics must ensure a stable mounting surface, adequate clearance, and access to a dedicated power outlet. |
| 4. What is the standard warranty coverage for the Carl Zeiss endodontic microscope, and are extended service plans available? | The Carl Zeiss endodontic microscope comes with a standard 3-year comprehensive warranty covering parts, labor, and optical performance. This includes preventive maintenance visits at 6, 18, and 30 months. Extended service agreements (ESA) are available for up to 5 additional years, offering priority response (within 48 business hours), remote diagnostics, and coverage for accidental damage. ESAs must be purchased within 90 days of equipment commissioning. |
| 5. How are firmware updates and technical support handled post-installation under the warranty? | Carl Zeiss provides over-the-air (OTA) firmware updates via the integrated Zeiss Connect Module, ensuring the microscope remains compliant with the latest imaging algorithms and safety protocols. Technical support is available 24/7 through the Zeiss Dental Support Hub (phone, email, and remote login). Warranty-covered clinics receive automatic update notifications and free remote assistance. On-site support is dispatched if remote resolution is not feasible, with response times guaranteed within 72 hours under standard warranty terms. |
Need a Quote for Carl Zeiss Endodontic Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


