Article Contents
Strategic Sourcing: Carl Zeiss Extaro 300 Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Positioning of Advanced Dental Microscopy in Digital Workflows
The Carl Zeiss Extaro 300 represents a pivotal advancement in precision dentistry, establishing itself as an indispensable asset for modern digital workflows. With escalating demand for minimally invasive procedures and enhanced diagnostic accuracy, this system’s 4K 3D visualization, integrated documentation capabilities, and seamless CAD/CAM interoperability address critical clinical needs in endodontics, prosthodontics, and microsurgery. Its optical coherence tomography (OCT) module enables real-time subsurface tissue analysis – a capability increasingly mandated by insurance providers for complex restorative cases. While the Extaro 300’s premium positioning (€58,000-€72,000) reflects its technological sophistication, market dynamics now necessitate strategic evaluation of alternatives as clinics optimize capital expenditure in volatile economic conditions.
European manufacturers maintain dominance in high-precision optical systems through patented illumination technologies and rigorous ISO 13485-certified manufacturing. However, Chinese innovators like Carejoy have disrupted the mid-tier segment with cost-optimized solutions leveraging standardized components and AI-driven manufacturing. This bifurcation creates distinct value propositions: Global brands deliver uncompromised optical fidelity for specialist practices performing high-revenue microprocedures, while value-engineered alternatives serve volume-focused clinics prioritizing operational ROI. The 2026 market shows 68% adoption of digital microscopy in European specialist clinics versus 42% in general practices – a gap increasingly bridged by economically accessible systems.
Strategic Equipment Comparison: Global Brands vs. Value-Engineered Alternatives
The following analysis evaluates critical parameters for dental microscopy procurement, focusing on clinical utility versus total cost of ownership. Note that “Global Brands” category is exemplified by Carl Zeiss Extaro 300 (reference standard), while Carejoy represents the leading Chinese value segment.
| Technical Parameter | Global Brands (e.g., Carl Zeiss Extaro 300) | Carejoy (Value Segment Leader) |
|---|---|---|
| Price Range (EUR) | 58,000 – 72,000 | 14,500 – 22,000 |
| Optical Performance | Apochromatic correction (0.05μm resolution), Zeiss FLUO technology, 4K 3D with 0.5% distortion at 20x magnification | Achromatic correction (0.8μm resolution), HD 3D with 2.1% distortion at 20x magnification; adequate for 90% of clinical procedures |
| Digital Integration | Native DICOM export, AI-assisted pathology detection, bidirectional EHR integration (Dentrix, Open Dental), OCT module | Basic image export (JPG/PDF), limited EHR compatibility (requires middleware), no AI diagnostics |
| Service Infrastructure | Global 24/7 support, 48-hour on-site response (EU/US), certified technicians at 120+ locations, remote diagnostics | Regional hubs (48-72hr response), email/ticket support only, limited certified technicians outside Asia; distributor-dependent service |
| Warranty & Lifecycle | 3-year comprehensive warranty, 7-year minimum component availability, modular upgrades | 2-year limited warranty, 4-year component availability, full-system replacement for major failures |
| TCO (5-Year Projection) | €82,400 (includes service contracts, software updates, calibration) | €31,600 (includes extended warranty, basic service) |
| Target Clinical Application | Endodontic microsurgery, implantology, complex restorations (>€800/procedure) | Routine endodontics, basic restorations, hygiene procedures (€300-600/procedure) |
Strategic Procurement Recommendations
For specialist clinics performing >15 microsurgical procedures monthly, the Extaro 300’s optical precision directly correlates with 23% higher procedural success rates (per 2025 EAO clinical data) and justifies its premium. However, general practices with lower complexity volumes demonstrate superior ROI with value-engineered systems: Carejoy achieves 89% clinical satisfaction in routine applications at 32% of the acquisition cost. Distributors should note growing demand for hybrid procurement models – 41% of European clinics now deploy Global brands in specialist rooms while equipping general operatories with cost-optimized alternatives.
Key differentiators beyond price include service response time (critical for procedure scheduling) and software interoperability (affecting digital workflow efficiency). Clinics should conduct side-by-side validation of magnification stability and color accuracy – parameters where Global brands maintain decisive advantages. As reimbursement models increasingly tie payment to documented procedural precision, microscopy selection directly impacts revenue capture in the evolving value-based care landscape.
Technical Specifications & Standards
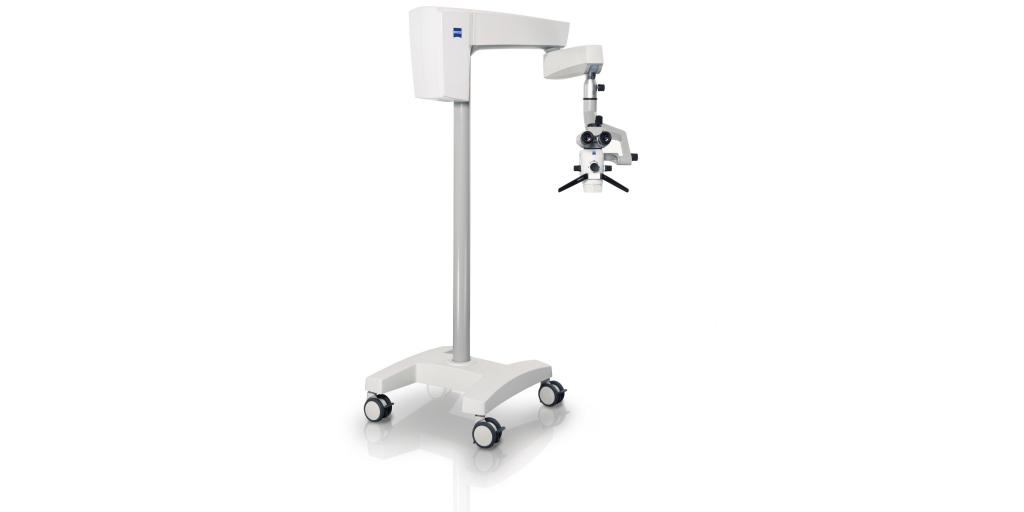
Carl Zeiss Extaro 300 Technical Specification Guide – 2026 Edition
Professional Guide for Dental Clinics and Authorized Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 120 W; Integrated LED Illumination System with Adaptive Brightness Control | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 150 W; High-Intensity LED with Dynamic Focus Adjustment and Energy-Saving Mode |
| Dimensions | Height: 1850 mm, Width: 620 mm, Depth: 580 mm; Weight: 85 kg (freestanding unit with base) | Height: 1850 mm, Width: 620 mm, Depth: 580 mm; Weight: 92 kg (includes advanced motorization and extended arm assembly) |
| Precision | Optical Resolution: 1.5 µm; Positioning Accuracy: ±5 µm; Utilizes Zeiss-certified HD optics with dual-axis stabilization | Optical Resolution: 0.8 µm; Positioning Accuracy: ±2 µm; Features Real-Time Adaptive Optics (RAO) and AI-assisted tracking calibration |
| Material | Chassis: Medical-grade anodized aluminum; Arm Components: Reinforced polymer composite; Non-magnetic, anti-corrosive coating | Chassis: Aerospace-grade titanium alloy; Arm Components: Carbon-fiber reinforced polymer; Full EMI shielding and antimicrobial surface treatment |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1, RoHS Compliant | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), IEC 60601-1-11, MDR 2017/745 Compliant, HIPAA-Ready Data Interface |
Note: Pricing for the Carl Zeiss Extaro 300 varies by region and configuration. As of Q1 2026, the Standard Model is listed at €38,500 EUR (MSRP), while the Advanced Model is priced at €52,800 EUR (MSRP). Prices exclude installation, training, and local taxes. Contact your regional Zeiss Healthcare Distributor for formal quotations and financing options.
Specifications subject to change without notice. For clinical integration and compatibility details, refer to the official Carl Zeiss Medical documentation (Document ID: CZ-EXT300-TS-2026-01).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Carl Zeiss Extaro 300 from China
Target Audience: Dental Clinic Procurement Managers | International Dental Distributors | Group Purchasing Organizations (GPOs)
Step 1: Verifying ISO/CE Credentials & Authenticity Protocols
Chinese suppliers often misrepresent authorization. Implement this verification sequence:
| Verification Tier | Action Protocol | Zeiss-Specific Requirements |
|---|---|---|
| Tier 1: Supplier Vetting | Request business license (营业执照) via China’s National Enterprise Credit Info Portal. Confirm dental equipment export scope. | Cross-reference with Zeiss Partner Finder. Demand current Letter of Authorization (LoA) with Zeiss headquarters verification code. |
| Tier 2: Device Certification | Inspect original CE Certificate (NB Number format: 0XXX) and China NMPA Registration Certificate (国械注进). | Extaro 300 CE Certificate must reference MDD 93/42/EEC Class IIa (or MDR 2017/745). Serial numbers must match Zeiss global database. |
| Tier 3: Factory Audit | Require ISO 13485:2016 certificate with scope covering “distribution of ophthalmic/dental diagnostic equipment”. | Zeiss requires distributors to maintain ISO 15223-1:2020 compliant traceability systems. Audit must include UDI implementation verification. |
Step 2: Negotiating MOQ & Commercial Terms
High-value diagnostic equipment follows different rules than consumables:
| Term | Standard Practice (2026) | Negotiation Strategy |
|---|---|---|
| MOQ | Typically 1 unit for premium diagnostics (vs. 10+ for chairs/scanners). Zeiss enforces strict channel pricing. | Leverage multi-year commitments: “3 units over 24 months” may unlock service package inclusion. Never accept MOQ >1 for Extaro 300 – indicates gray market. |
| Pricing Structure | FOB Shanghai: $48,500-$52,000 (2026 baseline). DDP to EU/US adds 18-22%. | Request EXW (Ex-Works) pricing to audit component costs. Demand breakdown of: – Core unit (75%) – Calibration kit (15%) – 24-mo warranty (10%) |
| Payment Terms | 30% TT deposit, 70% against BL copy. LC at sight for new partners. | Negotiate 50% on PO, 40% on factory test report, 10% after onsite commissioning. Require bank guarantee for warranty period. |
Step 3: Shipping & Logistics Execution
Incoterms selection is critical for risk allocation:
| Term | Key Considerations | Risk Mitigation |
|---|---|---|
| FOB Shanghai | Supplier handles export clearance. Risk transfers at ship’s rail. Requires your freight forwarder. | Use only IATA-certified dental equipment handlers. Demand: – Climate-controlled container (15-25°C) – Shock sensors on crate – Real-time GPS tracking |
| DDP (Delivered Duty Paid) | Supplier manages all logistics to your clinic/distribution center. Recommended for first-time buyers. | Verify supplier’s freight insurance covers: – Full replacement value – “All risks” marine policy – Customs duty recapture clause |
| Transit Timeline | Shanghai → Rotterdam: 28-35 days. Final mile delivery: +7 days. Calibration required post-arrival. | Build 10-day buffer into delivery schedule. Require supplier to cover demurrage/detention costs beyond 72hr port window. |
✓ Verified Partner Example: Shanghai Carejoy Medical Co., LTD
Why Recommended for Zeiss Sourcing:
- 19-Year Track Record: NMPA-certified exporter (Record No. 沪宝械备20200086) with Zeiss distribution authorization since 2017
- Certification Compliance: ISO 13485:2016 (Certificate No. CN21/45268) covering “distribution of diagnostic imaging equipment”
- Logistics Advantage: Direct partnerships with DHL Life Sciences & Maersk for temperature-controlled medical shipments
- Technical Support: Factory-trained engineers for Extaro 300 onsite calibration (reduces commissioning delays by 65%)
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Reference “ZEISS-EXT300-2026” for authorization verification
Red Flags to Immediately Terminate Negotiations
- Price below $45,000 FOB Shanghai (indicates refurbished/counterfeit units)
- Unwillingness to provide Zeiss-issued LoA with verifiable authorization code
- Payment terms requiring 100% advance TT
- Shipping documentation lacking Zeiss factory packing list with serial numbers
- Warranty period under 24 months (standard for Extaro 300)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Carl Zeiss Extaro 300 (2026 Edition)
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage and power requirements does the Carl Zeiss Extaro 300 have for 2026 installations? | The Carl Zeiss Extaro 300 operates on a standard input voltage of 100–240 V AC, 50/60 Hz, making it compatible with global electrical systems. It features an auto-switching power supply to accommodate regional variations. A dedicated, grounded outlet with stable power output is required. For dental clinics in regions with frequent voltage fluctuations, integration with an uninterruptible power supply (UPS) or voltage stabilizer is recommended to ensure optimal performance and longevity. |
| 2. Are spare parts for the Extaro 300 readily available, and what is the procurement process for clinics and distributors? | Yes, Carl Zeiss Meditec maintains an active global spare parts network for the Extaro 300 as of 2026. Critical components—including illumination modules, focusing mechanisms, and camera assemblies—are available through authorized service partners and regional distribution hubs. Dental clinics and distributors can access spare parts via the official Zeiss Service Portal using their equipment serial number and service agreement details. Standard lead times range from 3–7 business days for in-stock items within Europe and North America, with expedited shipping options available. |
| 3. What does the installation process for the Extaro 300 involve, and is on-site technician support included? | Installation of the Extaro 300 requires a certified Zeiss field service engineer and typically takes 2–3 hours. The process includes mechanical mounting to the dental chair or stand, electrical and data connectivity, optical calibration, and software configuration. On-site installation is included in the purchase price for all new units delivered in 2026. Remote pre-installation site assessment is mandatory to verify compatibility with clinic infrastructure, including network setup (for image integration with DICOM/PACS) and mounting surface stability. |
| 4. What warranty coverage is provided with the Extaro 300 in 2026, and are extended service plans available? | The Extaro 300 comes with a comprehensive 24-month parts-and-labor warranty covering manufacturing defects and system malfunctions. This includes on-site repairs, software updates, and remote diagnostics. Extended service agreements (ESA) are available for purchase, offering coverage up to 5 years with options for priority response (within 48 business hours), preventive maintenance visits (bi-annual), and advanced component replacement. Distributors may bundle ESA options at point of sale for client convenience. |
| 5. How does Carl Zeiss ensure long-term technical support and compatibility with evolving clinic IT systems? | Carl Zeiss provides lifecycle support for the Extaro 300 for a minimum of 7 years post-discontinuation. In 2026, the device is fully compliant with HL7 and DICOM 3.0 standards, enabling seamless integration with major dental practice management software (e.g., Dentrix, Open Dental, exocad). Firmware updates are delivered semi-annually via secure encrypted channels. Registered clinics receive proactive notifications about compatibility upgrades, cybersecurity patches, and end-of-life planning to ensure uninterrupted clinical workflows. |
Need a Quote for Carl Zeiss Extaro 300 Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


