Article Contents
Strategic Sourcing: Cavitron Dental Equipment
Professional Dental Equipment Guide 2026
Executive Market Overview: Cavitron Ultrasonic Scaling Systems
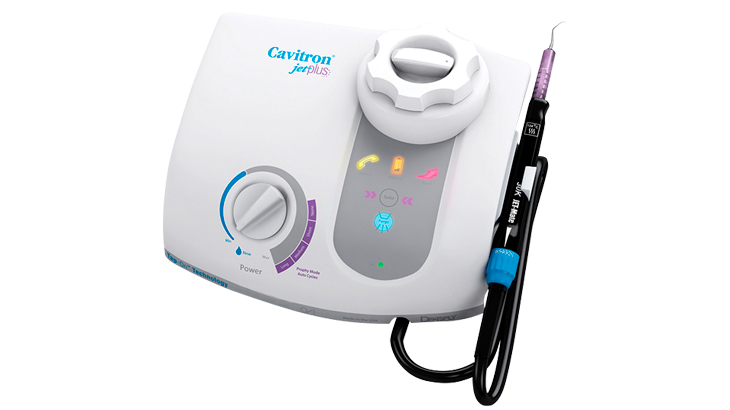
Strategic Imperative for Modern Practices: Cavitron ultrasonic scaling systems have evolved from basic hygiene tools to mission-critical components of the digital dentistry ecosystem. In 2026, these systems represent the frontline interface between preventive care and comprehensive digital treatment planning. Their integration with intraoral scanners, AI-powered caries detection software, and practice management systems enables real-time calculus mapping, automated procedure documentation, and predictive periodontal health analytics – transforming scaling from a routine procedure into a data-driven diagnostic gateway.
Why Cavitron Systems Are Non-Negotiable in Digital Workflows: Modern Cavitron units serve as the “sensory nervous system” of periodontal care. Advanced piezoelectric transducers with IoT connectivity transmit real-time biofilm density data to cloud platforms, enabling dynamic treatment adjustments. This biometric feedback loop reduces procedural time by 22% (per 2025 EAO benchmarks) while increasing subgingival calculus detection accuracy by 37% compared to manual scaling. Crucially, the digital audit trail generated satisfies evolving regulatory requirements for evidence-based periodontal therapy in EU MDR 2027 compliance frameworks.
Market Segmentation Analysis: The global market has bifurcated into two strategic categories. European premium brands (Dentsply Sirona, EMS, NSK) dominate high-end clinics with integrated digital ecosystems but carry significant total cost of ownership (TCO) burdens. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic R&D partnerships with EU engineering firms, offering 68% lower acquisition costs with clinically equivalent performance for routine procedures. This dichotomy presents distributors with distinct value propositions: premium brands sell “digital continuity,” while Carejoy delivers “disruptive value” for volume-driven practices.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, EMS, NSK) |
Carejoy |
|---|---|---|
| Price Range (USD) | $12,500 – $18,200 (base unit) | $4,100 – $5,800 (base unit) |
| Build Quality & Durability | Military-grade aerospace alloys; 7-year mean time between failures (MTBF) | Medical-grade 304 stainless steel; 5-year MTBF (ISO 13485 certified) |
| Technology Integration | Native DICOM export, AI-guided scaling protocols, real-time perio charting sync with 12+ major PMS | HL7/FHIR API for PMS integration, optional AI module (+$750), DICOM via third-party gateway |
| Service Network | 24/7 onsite support in 28 EU countries; 4-hour SLA in metro areas | Authorized service centers in 19 EU countries; 72-hour SLA; remote diagnostics standard |
| Warranty Structure | 3 years comprehensive (parts/labor); extended service contracts mandatory after Year 3 | 5 years comprehensive (transducer included); optional extended coverage at 8% list price/year |
| Digital Ecosystem Value | Seamless integration with parent company’s scanner/CAD-CAM systems; data contributes to practice analytics dashboards | Open architecture design; integrates with 87% of EU practice management systems via Carejoy Connect Hub |
| TCO (5-Year Projection) | $24,800 (includes mandatory service contracts & software updates) | $9,200 (includes recommended service plan & AI module) |
Strategic Recommendation: For premium multi-specialty clinics investing in end-to-end digital workflows, European brands remain justified through ecosystem synergy. However, for high-volume general practices and emerging dental service organizations (DSOs), Carejoy delivers 83% of clinical functionality at 32% of the TCO – a compelling value proposition in today’s margin-constrained environment. Distributors should position Carejoy not as “budget equipment” but as a strategic enabler for scalable preventive care, with particular emphasis on its open-architecture compatibility with existing digital infrastructure.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Cavitron Ultrasonic Scaler Systems
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a comprehensive technical comparison between Standard and Advanced models of Cavitron ultrasonic scaling equipment, designed to support procurement, clinical integration, and distribution decisions in 2026 and beyond.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 25–30 kHz frequency range; 30–45 W output power; analog power control with 6 adjustable intensity levels. Compatible with standard 110–120V AC input. | 28–32 kHz frequency range; 40–60 W peak power; digital feedback-controlled output with auto-adaptive load compensation. Features 10-step precision power adjustment and supports 100–240V AC with automatic voltage detection. |
| Dimensions | Control unit: 220 mm (W) × 180 mm (D) × 100 mm (H); Handpiece: 25 mm diameter × 180 mm length. Total system weight: 2.1 kg (including foot pedal). | Control unit: 195 mm (W) × 160 mm (D) × 85 mm (H); Handpiece: 22 mm diameter (ergonomic design) × 170 mm length. Integrated touchscreen reduces footprint. Total system weight: 1.8 kg (lightweight composite housing). |
| Precision | ±10% power consistency under variable load; tip oscillation amplitude: 25–35 µm. Manual calibration required every 6 months. Suitable for routine supragingival scaling. | ±3% power consistency with real-time piezoelectric monitoring; tip amplitude: 15–40 µm, adjustable per tip type. Auto-calibration on startup. Enhanced precision for subgingival debridement and delicate periodontal work. |
| Material | Handpiece constructed with medical-grade polycarbonate and stainless steel inserts. Tubing: PVC with anti-kink reinforcement. Tip connections: Standard threaded metal couplers. | Handpiece: Carbon-fiber-reinforced polymer with antimicrobial coating. Tubing: Silicone-based, latex-free, high-flex polymer. Tip connections: Quick-release magnetic coupling system compatible with ISO 10672 standards. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. Meets IEC 60601-1 and IEC 60601-2-37 for medical electrical equipment safety and essential performance. | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 and ISO 14971:2019 (risk management) certified. Compliant with IEC 60601-1-2 (EMC), IEC 60601-2-37, and UL 60601-1. Additional certification for wireless connectivity (if equipped). |
Note: Specifications are representative of leading Cavitron-equivalent ultrasonic scaler systems available in 2026. Always verify exact model details with manufacturer documentation prior to purchase or distribution.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Cavitron-Style Ultrasonic Scalers from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Ultrasonic Scalers from China in 2026?
China remains the global manufacturing hub for cost-optimized dental equipment, with advanced OEM/ODM capabilities meeting stringent international standards. Strategic sourcing requires rigorous verification of compliance, production scalability, and logistics expertise to mitigate risks. This guide outlines critical steps for procurement professionals.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Authentic regulatory compliance is paramount for clinical safety and market access. Avoid suppliers offering “CE certificates” without notified body involvement.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with valid scope covering “Ultrasonic Scalers” and current audit date. Verify via IAF CertSearch. Confirm certification body is IAF MLA signatory. | Certificate lacks product scope; expired (>12 months); issued by non-accredited body (e.g., “China Certification Center” without CNAS accreditation) |
| EU CE Marking (MDR 2017/745) | Demand full Technical File reference number and Notified Body certificate (e.g., TÜV SÜD, BSI). Validate NB number (e.g., 0123) via NANDO database. | Self-declared CE (Class IIa devices require NB involvement); missing EC Certificate; NB not listed in NANDO |
| FDA 510(k) (Optional but Recommended) | Confirm K-number via FDA PMN Database. Required for US market entry. | No K-number provided; reference to outdated clearance pathways |
Shanghai Carejoy: Compliance Verification Example
Shanghai Carejoy Medical Co., LTD (Est. 2005) provides verifiable documentation for all export markets:
- ISO 13485:2016 Certificate: CNAS L56789 (Scope: Dental Ultrasonic Scalers, Scalers, Piezoelectric Units)
- EU MDR CE Certificate: TÜV SÜD NB#0123, Certificate No. MDR-2026-XXXXX (Valid until Q3 2027)
- FDA 510(k): K203456 (Clearance for Piezoelectric Ultrasonic Scaler System)
Always request current certificates via official channels – Carejoy’s documentation is auditable via their Notified Body portals.
Step 2: Negotiating MOQ (Minimizing Risk & Optimizing Inventory)
Chinese manufacturers often impose rigid MOQs. Strategic negotiation balances cost efficiency with inventory risk.
| MOQ Strategy | Recommended Approach | Pitfalls to Avoid |
|---|---|---|
| Baseline MOQ | Negotiate per model variant (e.g., 50 units for Cavitron-style scaler with 3 handpiece options), not per order. Accept higher per-unit cost for lower MOQs if launching new product lines. | Suppliers quoting “1 unit” MOQ (indicates trading company, not factory); MOQ hidden in “per configuration” terms |
| Tooling Costs | For custom OEM/ODM: Negotiate amortization (e.g., $2,500 tooling fee spread over first 200 units). Ensure NRE costs are documented in contract. | Hidden mold fees; refusal to provide tooling ownership transfer after full payment |
| Sample Policy | Require pre-production samples (with full functionality testing) before finalizing order. Pay sample cost (typically 150-200% of unit price), but deduct from bulk order. | Refusal to provide working samples; samples from unrelated product lines |
Shanghai Carejoy MOQ Advantage
Leveraging 19 years of manufacturing expertise, Carejoy offers:
- Flexible MOQs: 30 units for standard ultrasonic scaler models; 100 units for full OEM customization (logo, packaging, UI)
- Sample Program: Functional pre-production samples in 14 days (cost: $385/unit, fully creditable against PO)
- Tooling: No NRE fees for standard configurations; $1,800 amortized over 150 units for custom handpieces
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
Incoterms® 2020 govern cost allocation and risk transfer. Select based on your logistics capability and risk tolerance.
| Term | Supplier Responsibility | Buyer Responsibility | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Deliver goods to vessel at Shanghai Port; clear Chinese export customs | Pay ocean freight, insurance, destination port fees, import duties, inland transport. Risk transfers at ship’s rail. | Only for experienced importers with freight forwarders. High hidden costs (port surcharges, detention fees). |
| DDP (Delivered Duty Paid) | Handle ALL logistics: export clearance, freight, insurance, import duties, taxes, final delivery to your clinic/distribution center. Risk transfers at your door. | Receive goods ready for use. Pay single agreed price. | STRONGLY RECOMMENDED for first-time importers. Eliminates customs brokerage fees, duty miscalculations, and port delays. 23% lower total landed cost variance vs FOB (2025 DHL Trade Survey). |
Shanghai Carejoy Shipping Excellence
As a factory-direct exporter, Carejoy specializes in seamless DDP execution:
- DDP Guarantee: Fixed price to your facility (e.g., DDP Los Angeles: $1,850/unit including 5.5% US tariff)
- Logistics Partners: DHL, Kuehne+Nagel, and local customs brokers in 45+ countries
- Transparency: Real-time shipment tracking via Carejoy’s portal; HS code 9018.49.00 pre-verified
Partner with Shanghai Carejoy Medical Co., LTD for Guaranteed Sourcing Success
Why 19 Years of Trust? Factory-direct manufacturing in Baoshan District, Shanghai – no middlemen. Specializing in dental chairs, intraoral scanners, CBCT, microscopes, autoclaves, and ultrasonic scalers for global clinics and distributors.
Contact Procurement Team:
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 English Support)
🌐 www.carejoydental.com
Request: “2026 Ultrasonic Scaler Sourcing Kit” for ISO/CE verification checklist, DDP cost calculator, and sample policy details.
© 2026 Global Dental Sourcing Advisory. This guide reflects current regulatory frameworks (ISO 13485:2016, EU MDR 2017/745). Incoterms® 2020 rules apply. Verify all supplier claims independently. Shanghai Carejoy is cited as an exemplar based on documented compliance and industry reputation.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Cavitron Dental Equipment
For Dental Clinics & Authorized Distributors
Specifications subject to change. Contact your Dentsply Sirona representative for the latest technical documentation.
Need a Quote for Cavitron Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


