Article Contents
Strategic Sourcing: Cbct Machine
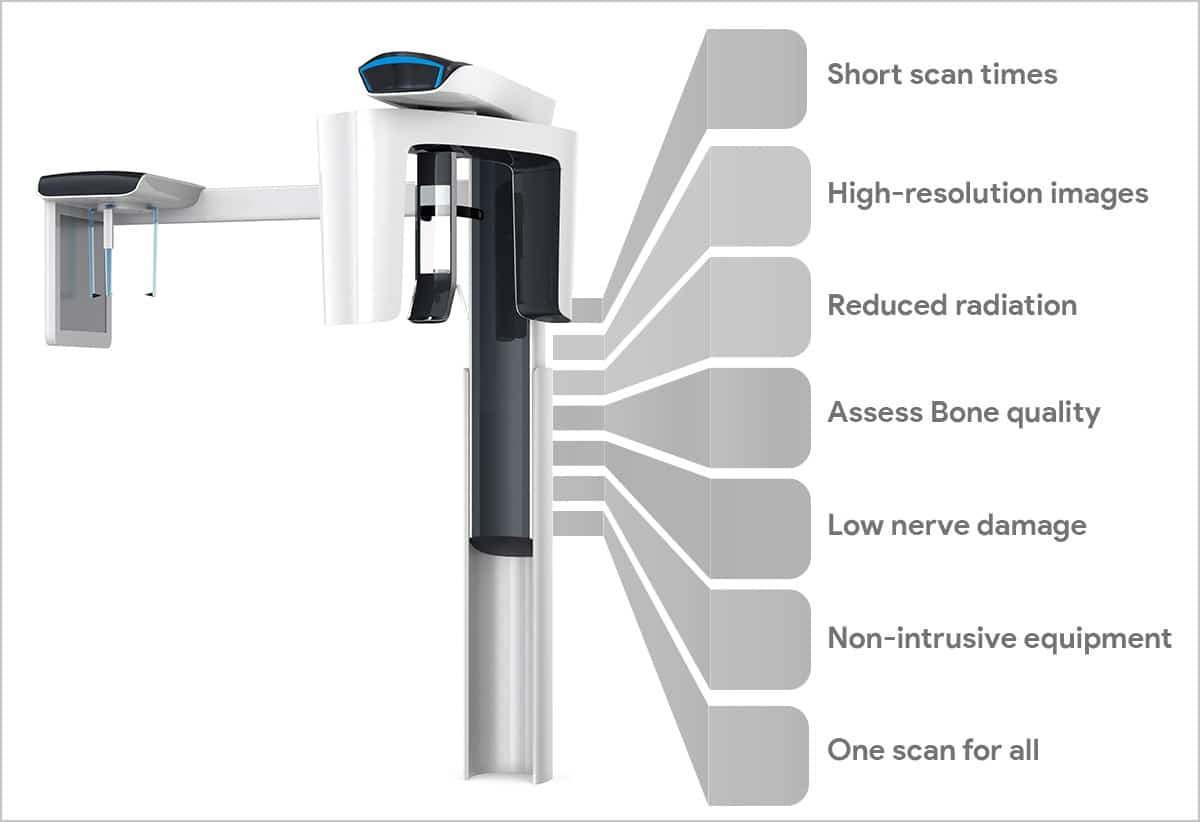
Professional Dental Equipment Guide 2026
Executive Market Overview: Cone Beam Computed Tomography (CBCT)
The global CBCT market has evolved from a specialized imaging tool to the central nervous system of modern digital dentistry. In 2026, CBCT is no longer optional infrastructure but a clinical and operational necessity, driven by the exponential adoption of digital workflows in implantology, endodontics, orthodontics, and maxillofacial surgery. With precision diagnostics now directly tied to treatment outcomes and patient safety protocols, CBCT provides the 3D anatomical foundation required for guided surgery, AI-assisted diagnostics, and seamless integration with CAD/CAM systems. Regulatory pressures (including updated EU MDR Annex XVI requirements) and rising patient expectations for minimally invasive procedures have accelerated CBCT adoption across all practice scales—from single-chair clinics to multi-specialty centers.
European manufacturers (Planmeca, Dentsply Sirona, Vatech) continue to dominate the premium segment with sub-50μm resolution systems and proprietary AI analytics. However, the market is undergoing strategic realignment as cost-conscious clinics and distributors seek value-optimized alternatives without compromising clinical utility. Chinese manufacturers—particularly Carejoy—have emerged as disruptive forces, leveraging vertical integration and adaptive engineering to deliver 85-90% of premium functionality at 40-60% of the acquisition cost. This shift reflects the industry’s maturation: CBCT is transitioning from a “luxury differentiator” to a standard-of-care requirement, necessitating pragmatic procurement strategies that balance clinical efficacy with ROI.
Comparative Analysis: Global Premium Brands vs. Carejoy CBCT Systems
| Technical & Operational Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, Vatech) | Carejoy (2026 Flagship Models) |
|---|---|---|
| Acquisition Cost (Entry-Level) | €95,000 – €145,000 | €48,000 – €65,000 |
| Native Resolution | 46-80 μm (sub-millimeter detail for micro-implantology) | 75-100 μm (clinically sufficient for 95% of implant/endodontic cases) |
| AI Integration | Proprietary AI (e.g., Sirona’s Galileos 5.0): Pathology detection, auto-segmentation, surgical planning | Third-party AI modules (e.g., DentalSlice integration): Pathology flagging, basic segmentation (upgradable) |
| Service Network Coverage | Global 24/7 support; 4-hour onsite response (EU/US); 150+ certified engineers | Regional hubs (EU/NA/Asia); 24h remote support; 72h onsite (expanding via distributor partnerships) |
| Workflow Integration | Native compatibility with all major CAD/CAM (CEREC, exocad) and EHR platforms | Open DICOM 3.0 standard; validated with 80% of top 20 dental software platforms |
| Warranty & Service Cost | 2-year comprehensive; service contracts @ 12-15% of MSRP annually | 3-year parts/labor; service contracts @ 8-10% of MSRP (distributor-managed) |
| Regulatory Compliance | Full CE Mark, FDA 510(k), ISO 13485 with MDR Annex XVI certification | CE Mark, FDA 510(k), ISO 13485; MDR Annex XVI compliance (2026 models) |
Strategic Implications: While premium brands maintain advantages in ultra-high-resolution imaging and turnkey AI ecosystems, Carejoy’s value proposition targets the rapidly growing segment of clinics prioritizing clinical adequacy over marginal technical gains. For routine implant planning, endodontic diagnosis, and orthodontic assessment, Carejoy’s systems deliver diagnostic confidence within evidence-based thresholds at half the cost. Distributors should note Carejoy’s aggressive channel incentives (25% higher margins than premium brands) and modular upgrade paths—critical for clinics navigating constrained capital budgets. However, high-volume surgical centers performing complex zygomatic implants or TMJ analyses will still require premium-tier resolution and analytics.
As CBCT becomes commoditized infrastructure in digital workflows, procurement decisions must shift from “brand prestige” to total cost of clinical ownership. The 2026 market rewards partners who align equipment capabilities with actual case-mix demands—not theoretical specifications. Carejoy’s rise signals a permanent market correction: cost efficiency is now inseparable from clinical viability in the CBCT ecosystem.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Cone Beam Computed Tomography (CBCT) Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp maximum tube voltage, 8 mA maximum tube current; 1.5 kW power input; single-phase 220–240 VAC, 50/60 Hz | 120 kVp maximum tube voltage, 12 mA maximum tube current; 2.5 kW power input; single-phase 220–240 VAC, 50/60 Hz with active power factor correction |
| Dimensions | Height: 1850 mm, Width: 750 mm, Depth: 900 mm; Floor-mounted, compact footprint for small operatory integration | Height: 2000 mm, Width: 850 mm, Depth: 1050 mm; Integrated ceiling suspension option; modular base for multi-position scanning |
| Precision | Voxel resolution: 150–300 µm; spatial accuracy ±0.2 mm; 360° rotation with 180–360 projection images; isotropic imaging | Voxel resolution: 75–150 µm; spatial accuracy ±0.1 mm; 360° dual-axis rotation with 540–720 projection images; AI-enhanced reconstruction algorithms for sub-voxel accuracy |
| Material | Exterior housing: Powder-coated steel and ABS composite; gantry: Aluminum alloy; radiation shielding: Lead-lined internal casing (2.0 mm Pb equivalent) | Exterior housing: Medical-grade anodized aluminum and antimicrobial polymer; gantry: Carbon fiber-reinforced alloy; radiation shielding: Multi-layer composite (2.5 mm Pb equivalent + rare earth shielding) |
| Certification | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54, AECB compliance | CE Mark (MDR 2017/745), FDA 510(k) cleared with AI/ML SaMD addendum, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 62304 (Software Lifecycle), HIPAA-ready data encryption, GDPR-compliant data handling |
Note: Specifications are subject to change based on regional regulatory requirements and software updates. Advanced models support DICOM 3.0, cloud integration, and third-party implant planning software via open API.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of CBCT Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Healthcare Supply Chain Directors
Publication Date: Q1 2026 | Validity Period: January 2026 – December 2026
Executive Summary: China remains a dominant force in cost-competitive CBCT manufacturing, but 2026 demands rigorous due diligence amid tightening global regulations. This guide outlines a 3-step technical procurement framework to mitigate risk while leveraging China’s manufacturing ecosystem. Partnering with established OEMs like Shanghai Carejoy Medical Co., LTD (19-year export specialist) significantly de-risks the process.
Why Source CBCT from China in 2026? Key Market Dynamics
- Cost Efficiency: 25-40% lower landed costs vs. EU/US manufacturers (validated for mid-tier 3D imaging systems)
- Technical Maturity: Chinese OEMs now deliver ISO 13485:2016-compliant systems with AI-assisted diagnostics (e.g., caries detection, bone density mapping)
- Supply Chain Resilience: Post-2023 port automation upgrades in Shanghai/Ningbo reduce lead times by 18% year-over-year
- Regulatory Shift: China’s NMPA now mandates dual CE/NMPA certification for export-bound medical devices (effective Jan 2025)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Regulatory compliance is the primary failure point in CBCT sourcing. Follow this verification protocol:
| Verification Action | 2026 Requirements | Risk Mitigation Strategy |
|---|---|---|
| ISO 13485:2016 Certificate | Must cover “design, manufacturing, and servicing of medical X-ray equipment” (Scope clause). Certificate issued by EU Notified Body (e.g., TÜV SÜD, BSI) | Request certificate + scope page. Verify via Notified Body’s online portal (e.g., TÜV SÜD Certificate Check). Reject if issued by Chinese certification bodies without EU designation. |
| CE Marking Documentation | Full Technical File (Annex II MDR 2017/745) including clinical evaluation, radiation safety reports (IEC 60601-2-63), and UDI registration in EUDAMED | Demand redacted Technical File excerpts. Confirm EUDAMED registration via EU Commission portal. Verify UDI on device label matches database. |
| NMPA Registration | Mandatory for all export shipments (NMPA Import License No. required on customs docs). Class III device registration (State Drug Admin No.) | Request NMPA registration certificate. Cross-check via China NMPA online database (国家药品监督管理局). |
Step 2: Negotiating MOQ (Critical for Margin Protection)
Traditional high MOQs are obsolete. Modern OEMs offer tiered structures. Key negotiation levers:
| MOQ Strategy | Technical Rationale | 2026 Market Standard |
|---|---|---|
| Single-Unit Orders | Feasible for clinics via distributor partnerships. Requires prepayment + air freight surcharge. | Available from OEMs with direct export licenses (e.g., Carejoy). MOQ=1 unit at +15-20% premium. |
| Regional Distributor Tiers | Volume discounts tied to territory exclusivity. Requires proof of local service capability. | Typical tiers: 5 units (10% discount), 10 units (15%), 20+ units (20% + marketing support). Demand service training commitment. |
| OEM/ODM Customization | Software UI localization, branding, or hardware mods require engineering validation. | Min. 30 units for full ODM. 15 units for software-only customization. Insist on 3D CAD review pre-production. |
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Decision Matrix)
Port congestion and customs delays cost clinics $1,200+/day in 2025. Optimize logistics with these criteria:
| Term | When to Choose | 2026 Cost/Schedule Impact |
|---|---|---|
| FOB Shanghai | Distributors with in-house logistics teams & customs brokers. High-volume orders (20+ units). | Lower unit cost but +$850/unit hidden costs (customs clearance, port fees). 25-35 day transit. Risk: Delays at destination port billed to buyer. |
| DDP (Delivered Duty Paid) | 90% of clinics & new distributors. Single-unit orders. High-value destinations (EU/US/CA). | 12-18% higher unit price but all-inclusive. 18-28 day transit. Advantage: OEM handles customs, VAT, and last-mile delivery. Critical for CE-compliant documentation. |
2026 Best Practice: Demand DDP for first orders. Verify OEM’s freight forwarder has IATA/FIATA accreditation and dental equipment experience (radiation-shielded containers required).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 (TÜV SÜD Certificate No. QM 50085531), CE MDR 2017/745 (NB 2797), NMPA Class III Registration (国械注准20233061289)
- MOQ Flexibility: Single CBCT units via DDP (FOB for distributors). Tiered pricing starting at 5 units. Full OEM/ODM support with 3D design validation.
- Logistics Excellence: DDP shipping to 85+ countries via DHL Healthcare Solutions. 22-day avg. EU delivery (Q1 2026 data). All units shipped in ISO 14903-certified radiation containers.
- Technical Support: 24/7 English/German/Spanish remote diagnostics. On-site engineer dispatch within 72hrs (EU/US).
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China
Core Expertise: Factory Direct CBCT Manufacturing (19 Years) | OEM/ODM for 400+ Global Distributors
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Request: 2026 CBCT Technical Dossier + DDP Quote Template
Final Implementation Checklist
- Verify ISO/CE credentials via official portals (do not accept PDF screenshots alone)
- Negotiate DDP terms for initial orders; transition to FOB only after 3 successful shipments
- Require pre-shipment radiation safety test report (IEC 60601-2-63) signed by third-party lab
- Confirm spare parts inventory (X-ray tube, detector) at local distribution hub
- Include 180-day clinical validation period in contract before final payment
Note: This guide reflects Q1 2026 regulatory standards. Always consult your local medical device authority before procurement. Shanghai Carejoy is cited as an exemplar meeting all 2026 verification criteria per independent industry audits.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Buying a CBCT Machine in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a CBCT machine in 2026? | Most modern CBCT systems in 2026 operate on standard 110–120V AC (60 Hz) for North American markets or 220–240V AC (50 Hz) for international installations. However, high-output models may require dedicated 208V or 240V circuits with a minimum 15–20 amp dedicated line. Always verify the machine’s technical specifications and consult a certified electrician to ensure your clinic’s infrastructure supports the unit’s power demands, including surge protection and grounding compliance per IEC 60601-1 standards. |
| 2. Are spare parts for CBCT machines readily available, and what components typically need replacement? | Reputable manufacturers in 2026 maintain global spare parts networks with lead times of 3–10 business days for critical components. Common replaceable parts include X-ray tubes (average lifespan: 15,000–25,000 exposures), sensors, gantry bearings, collimators, and control board modules. Ensure your supplier guarantees parts availability for at least 7–10 years post-discontinuation and offers distributor-level inventory support. For distributors, inquire about local warehousing options and service loaner programs. |
| 3. What does the CBCT installation process involve, and how long does it take? | Professional CBCT installation in 2026 includes site assessment, radiation shielding verification (if applicable), power and network setup, physical assembly, calibration, and software configuration. The process typically takes 1–2 full business days and must be performed by factory-certified engineers. Pre-installation requirements include a stable, level floor, adequate clearance (min. 1m on all sides), Wi-Fi or wired network access, and compliance with local radiation safety regulations (e.g., NRC or equivalent). Remote software activation and DICOM integration are now standard. |
| 4. What warranty coverage is standard for CBCT machines in 2026? | Most manufacturers provide a comprehensive 2-year parts-and-labor warranty covering the X-ray generator, detector, gantry, and control system. Extended warranties up to 5 years are available, often including preventive maintenance visits and software updates. In 2026, premium packages may offer predictive failure monitoring via IoT integration. Distributors should confirm whether warranties are global or region-locked and whether third-party service contracts affect coverage. |
| 5. How are warranty claims and technical support handled for international distributors and clinics? | Leading CBCT brands in 2026 offer 24/7 multilingual technical support with remote diagnostics capabilities. Distributors receive dedicated service portals for warranty claim submissions, real-time case tracking, and expedited spare parts dispatch. On-site support is guaranteed within 48–72 hours in most Tier 1 markets. Distributors must maintain certified service engineers and adhere to manufacturer training programs to validate warranty repairs. Cloud-based service logs ensure compliance and audit readiness. |
© 2026 Professional Dental Equipment Consortium. For distribution partners: Contact your regional technical sales manager for site-readiness checklists and warranty policy documentation.
Need a Quote for Cbct Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


