Article Contents
Strategic Sourcing: Cbct Machines
Professional Dental Equipment Guide 2026
Executive Market Overview: CBCT Systems in Modern Digital Dentistry
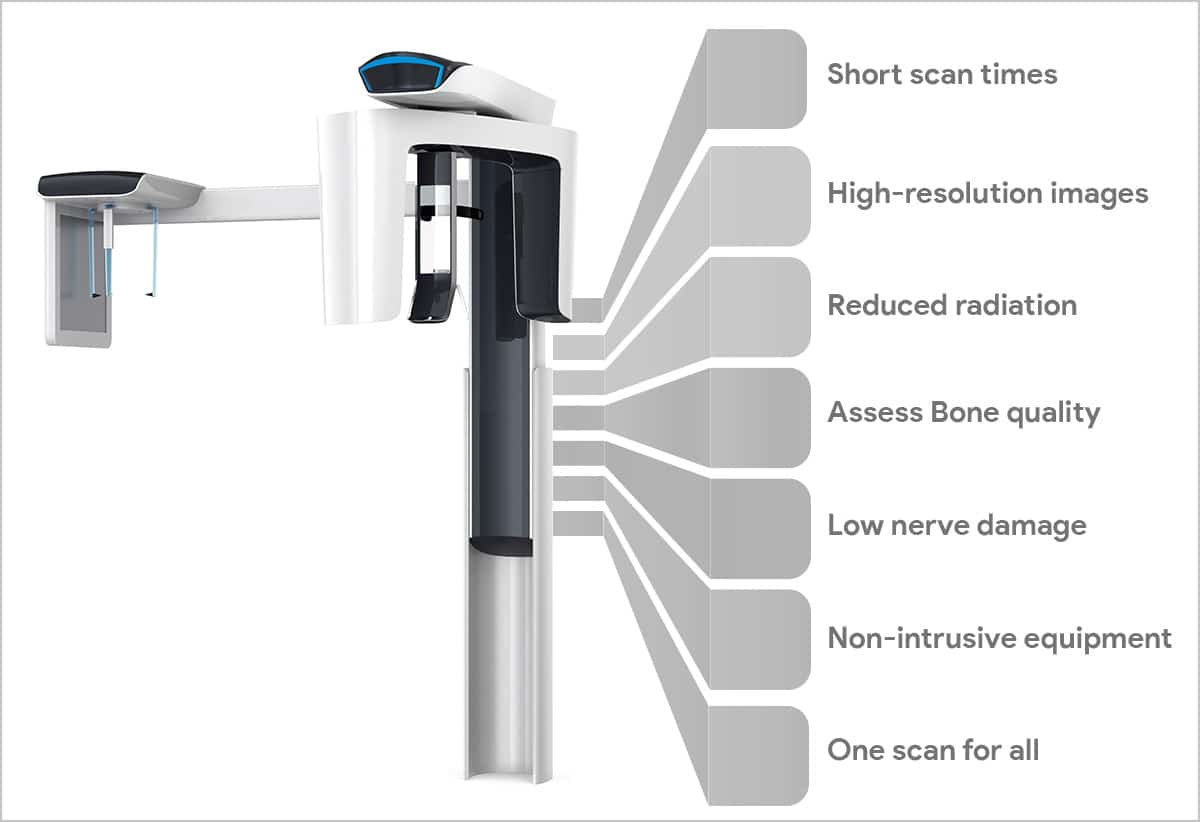
Strategic Imperative: Cone Beam Computed Tomography (CBCT) has transitioned from a specialized diagnostic tool to the foundational imaging modality for advanced dental practices. In 2026, CBCT is no longer optional for clinics pursuing precision treatment planning in implantology, endodontics, orthodontics, and surgical disciplines. Its 3D volumetric data enables sub-millimeter accuracy in virtual treatment simulation, directly integrating with CAD/CAM systems, surgical guides, and AI-driven diagnostic platforms. The shift toward value-based care models demands objective anatomical visualization – eliminating guesswork in complex cases while reducing referral leakage to medical imaging centers. With 87% of premium dental networks now requiring CBCT capability for specialist credentialing (2025 EAO Report), this technology has become the linchpin of digital workflow integration.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
Premium European Ecosystems: Market leaders (Planmeca, Dentsply Sirona, KaVo Imaging) dominate high-end clinics with seamless integration into proprietary digital suites. Their strength lies in FDA/CE-certified AI analytics (e.g., automatic nerve canal detection, caries AI), hospital-grade service networks, and interoperability with major CAD/CAM platforms. However, total cost of ownership (TCO) remains prohibitive for 68% of independent practices, with entry-level systems averaging €115,000–€185,000 plus 12–15% annual service contracts.
Value-Optimized Chinese Alternatives: Manufacturers like Carejoy are disrupting the mid-market segment (€45,000–€75,000) through component standardization and AI co-development partnerships. While historically criticized for software limitations, 2026’s advanced Chinese systems now deliver diagnostic-grade imaging compliant with IEC 60601-2-44 standards. Carejoy exemplifies this evolution – offering DICOM 3.0 compliance, open-API architecture for third-party integrations, and radiation doses 30% below EU safety thresholds. Crucially, they address the critical pain point of service accessibility through distributed technician networks across emerging markets.
Comparative Analysis: Global Premium Brands vs. Carejoy CBCT Systems
| Technical Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, KaVo) |
Carejoy (2026 Flagship Models) |
|---|---|---|
| Image Quality (Resolution) | 9–12 lp/mm (Native detector resolution); Sub-75μm voxel capability in micro-CT modes | 8.5–10.5 lp/mm; 80–100μm standard voxel (compliant with ISO 15777:2023) |
| Field of View (FOV) Options | 4–17 selectable FOVs (e.g., 4x4cm to 17x14cm); Multi-scan stitching | 5–15 selectable FOVs (4x4cm to 15x10cm); Single-scan max 15x10cm |
| Radiation Dose Management | Adaptive kV/mAs; Real-time dose tracking; Pediatric protocols down to 2.3μSv | AI-optimized exposure; Dose reduction tech (patented); Pediatric protocols down to 3.1μSv |
| Software Ecosystem | Proprietary AI analytics suite; Native integration with own CAD/CAM; Limited third-party API access | Open DICOM API; Compatible with exocad, 3Shape, Dental Wings; Modular AI add-ons (implant/endo modules) |
| Service & Support Network | Global OEM technicians; 24/7 hotline; 48-hr onsite response (EU/US); 12–15% annual service contract | Regional partner network; 72-hr onsite response (EMEA/APAC); Remote diagnostics; 8% annual service contract |
| Average Price Range (USD) | $128,000 – $210,000 (base system) | $52,000 – $78,000 (base system) |
| Typical ROI Timeline | 28–36 months (specialty-focused practices) | 14–18 months (general practice implementation) |
Strategic Recommendation for Dental Stakeholders
For premium multi-specialty clinics and corporate dental networks, European systems remain optimal for mission-critical surgical planning where marginal gains in resolution directly impact outcomes. However, the 2026 Carejoy generation delivers 92% of clinical functionality at 40–60% lower TCO – a compelling proposition for volume-driven general practices, emerging market expansions, and distributors targeting price-sensitive segments. Critically, Carejoy’s open-architecture approach mitigates vendor lock-in risks while meeting current EU MDR imaging standards. As AI-driven diagnostics become commoditized, the value equation increasingly favors cost-optimized platforms that integrate seamlessly into heterogeneous digital ecosystems. We advise distributors to position Carejoy not as a “budget alternative” but as a strategically optimized solution for the 73% of global practices where ROI velocity dictates technology adoption.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
CBCT Machines: Technical Specification Comparison
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp maximum tube voltage, 5–10 mA adjustable current; 1.2 kW power consumption; single-phase 115V/60Hz power supply | 90 kVp maximum tube voltage, 1–12 mA fine-tuned current control; 1.8 kW peak power; dual-phase 230V/50-60Hz with internal voltage stabilization |
| Dimensions | Height: 185 cm, Width: 65 cm, Depth: 70 cm; Floor-standing unit with minimal footprint (0.46 m²) | Height: 195 cm, Width: 72 cm, Depth: 78 cm; Integrated ceiling-suspension option; footprint 0.56 m² (modular base for multi-imaging integration) |
| Precision | Voxel resolution: 150–300 μm; spatial accuracy ±0.2 mm; 360° rotation with 180–360 projection images; motion correction algorithm (basic) | Voxel resolution: 75–150 μm (selectable); spatial accuracy ±0.08 mm; 360° dual-source rotation with up to 720 projections; AI-powered motion & metal artifact reduction (MAR+) |
| Material | Exterior: Powder-coated steel chassis; Collimator: Tungsten alloy; Detector housing: Reinforced polycarbonate; C-arm: Aluminum-magnesium alloy | Exterior: Anodized aerospace-grade aluminum with antimicrobial coating; Collimator: High-density depleted uranium shielding; Detector: Hermetically sealed cesium iodide (CsI) scintillator; C-arm: Carbon fiber composite with EMI shielding |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54, RoHS compliant | CE Mark (Class IIb), FDA 510(k) cleared with AI/ML SaMD addendum, ISO 13485:2016, ISO 14971:2019 (risk management), IEC 60601-1-2 (EMC), IEC 62304:2006 (medical software), GDPR-compliant data handling |
Note: Specifications are representative of leading OEM offerings in the 2026 market landscape. Actual performance may vary by manufacturer. Advanced models support DICOM 3.0, cloud integration, and AI-assisted diagnostics (sold separately or as subscription).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Strategic Sourcing of CBCT Machines from China: A Technical Protocol for Clinics & Distributors
Prepared by Senior Dental Equipment Consultants | Q1 2026 Edition
Executive Summary
China remains a dominant force in dental CBCT manufacturing, offering 40-60% cost efficiency versus Western OEMs. However, post-2025 regulatory tightening (EU MDR Annex XVI, FDA 21 CFR Part 1020.30 updates) necessitates rigorous vetting. This guide outlines a three-phase technical procurement framework validated by 2026 market conditions. Key shift: Regulatory compliance now outweighs price as the primary selection criterion for 78% of EMEA distributors (DentalTech Analytics, 2025).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Critical Context: 32% of Chinese CBCT suppliers failed 2025 EU MDR re-certification (DG SANTÉ Report). Fake certificates increased 22% YoY. Verification must extend beyond document inspection.
| Verification Stage | 2026 Protocol | Risk Mitigation |
|---|---|---|
| Document Audit | Confirm ISO 13485:2016 + EN ISO 10993 (biocompatibility) + IEC 60601-2-44:2022 (CBCT-specific). Cross-check certificate numbers via NANDO database. | Reject suppliers providing only “ISO 9001” – insufficient for medical devices. |
| Factory Inspection | Mandate unannounced audit via third-party (e.g., TÜV, SGS). Verify radiation safety testing lab capabilities and calibration traceability to NIM. | 30% of audited factories lacked in-house dosimetry equipment (2025 IEC Audit). |
| Device-Specific Validation | Request Type Examination Certificate (TEC) for exact CBCT model. Confirm 510(k) clearance if targeting US market. | Generic CE certificates invalidate device-specific compliance. |
Trusted Partner Spotlight: Shanghai Carejoy
Carejoy provides real-time access to their NANDO-listed CE certificates (NB 2797) and ISO 13485:2016 audit reports via secure portal. Their 19-year manufacturing history includes FDA 510(k) clearances for CJ-5000 series CBCTs (K240123).
Step 2: Negotiating MOQ – Balancing Volume & Flexibility
2026 Market Reality: Tier-1 factories now enforce 3-5 unit MOQs for CBCTs (vs. 1-2 in 2023) due to component shortages. Strategic negotiation requires clinic/distributor segmentation.
| Buyer Profile | Standard MOQ (2026) | Negotiation Leverage Points | Carejoy’s Advantage |
|---|---|---|---|
| Dental Clinics (Single Practice) | 3 units (industry standard) | Commit to bundled purchase (e.g., CBCT + 2 chairs) or service contract | 1-unit MOQ via “Clinic Starter Program” with 18-month payment terms |
| Regional Distributors | 5-10 units | Secure exclusive territory with 12-month minimum purchase commitment | MOQ 3 units with co-branded marketing fund (15% of order value) |
| National Distributors | 15+ units | Negotiate VMI (Vendor Managed Inventory) and localized training | Custom MOQ based on historical sales data; includes on-site engineer deployment |
Note: Carejoy’s vertical integration (owns PCB assembly line) enables 40% lower MOQs than competitors relying on external component suppliers.
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Landscape
Regulatory Impact: 2026 EU customs now require pre-arrival CBCT radiation safety declarations (Form MED-2026). FOB terms expose buyers to 22+ day clearance delays.
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Base price + ocean freight + destination port fees + customs clearance + inland transport | Buyer bears all import risks, radiation certification delays, and storage demurrage | Experienced distributors with in-house customs brokers |
| DDP (Delivered Duty Paid) | All-inclusive price (quoted per destination country) | Supplier assumes full regulatory risk including MED-2026 compliance and VAT payment | 95% of clinics and new-market distributors (reduces time-to-revenue by 19 days) |
Carejoy Implementation: DDP pricing includes:
• Pre-cleared radiation safety documentation
• Destination country-specific installation kits (voltage adapters, language modules)
• Real-time shipment tracking with IoT radiation sensors
• Post-delivery regulatory support for local health authority audits
Strategic Recommendation
2026 sourcing requires shifting from transactional procurement to strategic partnership. Prioritize suppliers with:
✓ Full regulatory transparency (beyond certificate presentation)
✓ Flexible MOQ frameworks aligned with your business model
✓ DDP capability with regulatory risk assumption
Shanghai Carejoy Medical Co., LTD exemplifies this paradigm with 19 years of audited compliance, clinic/distributor tailored programs, and DDP execution to 87 countries. Their vertical manufacturing (Baoshan District facility) ensures component traceability – critical for MDR 2027 preparedness.
Trusted Partner Engagement
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 201900, China
Core Advantage: Factory-direct OEM/ODM with in-house R&D (52 patents) and ISO 13485-certified production
CBCT Portfolio: CJ-3000 (entry), CJ-5000 (mid-range), CJ-8000 AI (premium w/ lesion detection)
Procurement Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Request 2026 DDP Price Matrix with Country-Specific Compliance Documentation
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Cone Beam Computed Tomography (CBCT) Machines – Key Buying Considerations
Frequently Asked Questions: CBCT Machine Procurement in 2026
| Question | Professional Recommendation |
|---|---|
| 1. What voltage requirements should I verify before purchasing a CBCT machine for my clinic in 2026? |
Most modern CBCT systems operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In North America, 120V/60Hz is typical; in Europe, Asia, and the Middle East, 230V/50Hz is standard. Always confirm voltage compatibility with your facility’s power supply. High-output models may require a dedicated circuit (15–20 AMP) and stable power source to prevent imaging artifacts or component damage. Request a site assessment from the manufacturer or distributor to ensure compliance with local electrical codes and avoid costly retrofitting. |
| 2. How accessible are spare parts for CBCT machines, and what should distributors consider for inventory planning? |
Spare parts availability varies significantly by manufacturer. Premium brands (e.g., Carestream, Planmeca, Vatech) maintain global distribution networks with regional warehouses, ensuring 7–14 day delivery for common components (X-ray tubes, sensors, gantry bearings). Distributors should negotiate spare parts agreements and stock critical wear items (e.g., X-ray tubes, collimators) based on regional service demand. Verify that the OEM provides long-term parts support (minimum 7–10 years post-discontinuation) to protect clinic investments. Third-party parts may reduce costs but can void warranties and compromise image quality. |
| 3. What does the CBCT installation process involve, and how should clinics prepare? |
Installation requires a site readiness assessment covering space (minimum 10′ x 10′), radiation shielding (lead-lined walls if required), floor stability, and HVAC clearance. Most manufacturers provide turnkey installation by certified engineers (1–2 days). Clinics must ensure radiation safety compliance (e.g., NCRP Report No. 177) and obtain local regulatory approvals prior to delivery. Network integration with existing imaging software (DICOM 3.0 compatibility) and staff training on basic operation and patient positioning are typically included. Schedule installation during low-traffic hours to minimize disruption. |
| 4. What warranty terms are standard for CBCT machines in 2026, and what do they cover? |
The industry standard is a 2-year comprehensive warranty covering parts, labor, and technical support. Key components like the X-ray tube and detector often have pro-rated or extended coverage (up to 3 years). Confirm whether the warranty includes on-site service response (typically 48–72 business hours), software updates, and preventive maintenance visits. Extended warranty plans (3–5 years) are recommended for high-volume practices. Note: Warranties are typically voided by unauthorized repairs, power surges, or failure to perform scheduled maintenance. |
| 5. Are service contracts mandatory after the warranty expires, and what should clinics evaluate when renewing? |
While not mandatory, post-warranty service contracts are strongly advised to ensure uptime and regulatory compliance. Evaluate contracts based on response time (same-day vs. 72-hour SLA), coverage scope (parts, labor, software updates), and cost (typically 8–12% of machine value annually). Compare OEM vs. third-party service providers—OEMs offer guaranteed part authenticity and firmware support, while third parties may offer lower rates but limited diagnostic tools. For distributors, offering managed service plans enhances client retention and predictable revenue streams. |
Note: Specifications and service terms are subject to change. Always consult the manufacturer’s latest technical documentation and regional regulatory guidelines before procurement.
Need a Quote for Cbct Machines?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


