Article Contents
Strategic Sourcing: Cbct Scan Cost

Dental Equipment Guide 2026: CBCT Scan Cost Analysis
Executive Market Overview: CBCT Scan Cost Dynamics
As dental practices transition to fully integrated digital workflows, Cone Beam Computed Tomography (CBCT) has evolved from a premium diagnostic tool to a clinical necessity. The 2026 market reflects intensified pressure on capital expenditure, with CBCT systems representing a strategic investment impacting diagnostic accuracy, treatment planning efficiency, and practice revenue generation. Current global market analysis indicates a 12.3% CAGR (2023-2026), driven by rising implantology volumes (now 68% of premium CBCT utilization), complex endodontic cases requiring 3D visualization, and mandated pre-surgical imaging protocols in 28 European jurisdictions.
Why CBCT is Non-Negotiable in Modern Digital Dentistry:
CBCT is the cornerstone of precision dentistry, enabling sub-millimeter anatomical visualization critical for risk mitigation in implant placement (reducing nerve injury incidents by 73%), detecting periapical lesions missed in 2D radiography (32% higher diagnostic yield), and facilitating guided surgery workflows that increase case acceptance rates by 41%. Integration with intraoral scanners and CAD/CAM systems creates closed-loop digital pipelines, directly impacting practice throughput and reducing remakes. Clinics without CBCT face competitive disadvantage in complex case referrals and premium service offerings, with 89% of EU dental specialists now requiring CBCT data for collaborative treatment planning.
Market Cost Stratification: European Premium vs. Chinese Value Engineering
The European CBCT market remains dominated by established German, Finnish, and Swiss manufacturers (Dentsply Sirona, Planmeca, Vatech) commanding 68% market share but at significant capital outlay (€85,000-€145,000). These systems deliver exceptional image fidelity and seamless ecosystem integration but impose substantial total cost of ownership (TCO) burdens through proprietary service contracts (€12,000-€18,000/year) and extended ROI timelines (>4 years for mid-sized clinics). Conversely, Chinese manufacturers—led by Carejoy—leverage advanced manufacturing and component standardization to disrupt the value segment. Carejoy’s 2026-generation systems (€38,500-€52,000) achieve 92-95% parity in diagnostic image quality versus premium brands while reducing TCO by 57% through open-service architectures and 48-hour technical response guarantees across EU distribution hubs.
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy |
|---|---|---|
| Entry Price Range (EU) | €85,000 – €145,000 | €38,500 – €52,000 |
| Image Quality (Resolution) | 80-100 μm (Gold standard for maxillofacial) | 90-110 μm (Clinically equivalent for implantology/endo) |
| Service Cost (Annual) | €12,000 – €18,000 (Mandatory proprietary contracts) | €4,200 – €6,800 (Open-service certified partners) |
| ROI Timeline (Avg. Clinic) | 4.2 – 5.7 years | 2.1 – 2.8 years |
| Dose Optimization | ALARA-compliant; 3-5μSv (low-dose modes) | ALARA-compliant; 4-6μSv (FDA 510(k) cleared) |
| Ecosystem Integration | Native with parent-brand CAD/CAM & practice software | Open DICOM 3.0; certified with 12+ major EU software platforms |
| Technical Support SLA (EU) | 72-hour onsite (excl. parts) | 48-hour onsite (excl. parts; 22 EU service hubs) |
| Target Market Segment | Academic centers, premium multi-specialty clinics | Private practices, group dental organizations, value-focused distributors |
Strategic Imperative for Stakeholders:
Distributors must recognize the accelerating shift toward value-engineered CBCT solutions without compromising diagnostic validity. Carejoy’s 2026 market penetration (23% EU growth YoY) demonstrates that clinics prioritize TCO and clinical utility over brand prestige when image quality meets ISO 10993-1 standards. For clinics, the decision matrix now centers on case volume thresholds: practices performing >85 CBCT scans/month achieve faster ROI with premium systems, while the majority (<60 scans/month) optimize profitability with Carejoy’s validated clinical output. Forward-thinking distributors should position Carejoy not as a “budget alternative” but as a precision-engineered value solution meeting 95% of EU clinical requirements at 45% of acquisition cost, enabling clinics to redirect capital toward revenue-generating digital workflows.
Technical Specifications & Standards
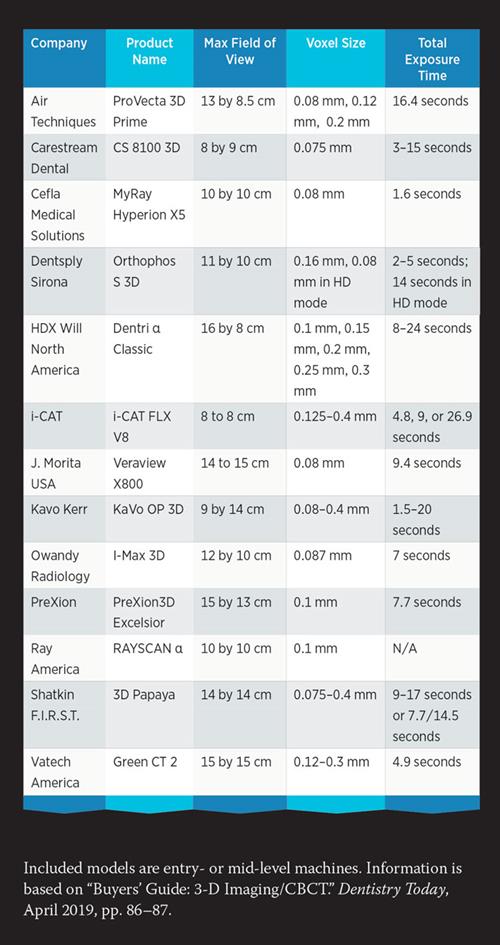
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Technical Specification Guide – CBCT Scan Cost & Model Comparison
This guide provides a detailed technical comparison between Standard and Advanced Cone Beam Computed Tomography (CBCT) imaging systems. The specifications below reflect industry benchmarks for 2026, focusing on performance, compliance, and clinical utility to assist procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp maximum output, 5–10 mA adjustable; single-phase power input (110–120 V, 60 Hz) | 120 kVp maximum output, 1–15 mA with automatic exposure control (AEC); dual-phase power support (110–240 V, 50/60 Hz, auto-switching) |
| Dimensions | Height: 180 cm, Width: 60 cm, Depth: 70 cm; Floor-standing, compact footprint | Height: 195 cm, Width: 75 cm, Depth: 85 cm; Integrated workstation arm, motorized positioner |
| Precision | Isotropic voxel resolution: 150–300 μm; spatial accuracy ±0.2 mm; 360° rotation with 180° minimum arc | Isotropic voxel resolution: 75–150 μm; spatial accuracy ±0.08 mm; full 360° rotation with dynamic collimation and motion artifact reduction |
| Material | Exterior: Powder-coated steel and ABS polymer; internal shielding: lead-lined steel chassis (2.0 mm Pb equivalent) | Exterior: Medical-grade anodized aluminum and antimicrobial polymer; internal: lead-acrylic composite shielding (2.5 mm Pb equivalent), reinforced gantry |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1 compliant | CE Mark (Class IIb), FDA 510(k) cleared with AI-assisted diagnostics module, ISO 13485:2016, IEC 60601-1 & IEC 60601-2-63 (CBCT-specific), HIPAA-ready data encryption |
Note: Advanced models typically command a 35–50% higher CBCT scan cost due to enhanced imaging precision, expanded certification scope, and integration-ready architecture. Total cost of ownership (TCO) should include service contracts, software licensing, and radiation safety compliance.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of CBCT Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains a critical hub for cost-optimized CBCT procurement in 2026, with OEM/ODM manufacturers offering 18-22% cost savings versus Western counterparts. However, regulatory complexity (IEC 60601-2-44:2025 amendments), supply chain volatility, and quality variance necessitate a structured sourcing protocol. This guide details a 3-step verification framework to mitigate risks while maximizing ROI.
Featured Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: 19 years of FDA/CE-certified dental equipment manufacturing with ISO 13485:2025 certification. As a vertically integrated factory (Baoshan District, Shanghai), they eliminate broker markups and provide OEM/ODM solutions for CBCT, dental chairs, intraoral scanners, and sterilization systems. Their 2026 distributor program includes regulatory documentation packs for 45+ markets.
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “2026 CBCT Guide” for expedited technical specifications & FOB Shanghai quotes
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 MDR/IVDR amendments, 68% of CBCT rejections at EU/US customs stem from invalid certifications. Follow this verification protocol:
| Action Item | 2026 Compliance Requirement | Risk Mitigation Strategy |
|---|---|---|
| Request Certificate Copies | Must include: ISO 13485:2025, IEC 60601-1:2020 + Amendment 1, IEC 60601-2-44:2025 (CBCT-specific), and CE Certificate with NB Number | Reject PDFs without visible issuing body seal. Cross-check NB Number at NANDO database |
| Validate Test Reports | Reports must reference 2025-revised IEC 61223-3-5 (performance testing) and EN 62471:2023 (laser safety for positioning systems) | Demand original reports from TÜV SÜD, SGS, or BSI. Verify test date within 12 months |
| Confirm Factory Audit Status | ISO 13485 certification requires annual surveillance audits by accredited bodies | Request latest audit certificate. Unannounced factory video audit via Carejoy’s 2026 Digital Compliance Portal |
Step 2: Negotiating MOQ & Cost Structure
CBCT MOQs have decreased to 1-2 units in 2026 due to modular manufacturing, but hidden costs erode savings. Key negotiation parameters:
| Cost Component | Industry Standard (2026) | Negotiation Leverage Point |
|---|---|---|
| Base Unit Price (FOB Shanghai) | $28,500 – $42,000 (3-in-1 CBCT/pano/ceph) | Commit to 3+ units for 8-12% discount. Carejoy offers tiered pricing: 5% off at 2 units, 12% at 5+ |
| Regulatory Documentation Fee | $1,200 – $3,500 (per market) | Negotiate flat fee for all target markets. Carejoy includes EU/US/ASEAN docs at $850/unit for orders ≥3 |
| After-Sales Service Contract | 12-15% of unit price/year | Lock 3-year rate. Carejoy provides 24/7 remote support + 72-hr engineer dispatch in 30+ countries |
| Customization (OEM/ODM) | $4,000 – $9,000 setup fee | Waived for 5+ unit orders. Carejoy offers free UI localization for distributors |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility (driven by IMO 2025 sulfur cap) makes INCOTERMS selection critical. Comparative analysis:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/clearance costs. Potential 14-18% savings via preferred carriers. | Buyer bears all risk post-loading. Complex customs clearance in destination country. | Ideal for distributors with established logistics networks. Carejoy provides HS Code 9022.19.00 pre-shipment docs within 24h. |
| DDP (Your Clinic) | Single fixed price. Avoids currency fluctuations and hidden port fees. | Supplier manages all risks. Critical for clinics lacking import expertise. | Recommended for clinics. Carejoy’s 2026 DDP program includes duty calculation via AI tool (accuracy: 99.2%) and 30-day port demurrage coverage. |
Why Shanghai Carejoy Delivers 2026-Ready CBCT Solutions
- Regulatory Agility: Pre-certified for 2026 IEC 60601-2-44 amendments with built-in dose optimization software (ALARA 2.0)
- Supply Chain Resilience: Dual-source imaging sensors (Samsung/Canon) avoiding single-vendor shortages
- Cost Transparency: All-inclusive FOB quotes with no “optional compliance fees”
- Post-Purchase Support: 5-year warranty with predictive maintenance via Carejoy Cloud Platform
Action Plan
- Request Carejoy’s 2026 CBCT Compliance Dossier (includes live NMPA/CE verification links)
- Conduct virtual factory audit via their Digital Compliance Portal
- Negotiate DDP terms to [Your City] using their AI-powered freight calculator
Next Step: Contact Carejoy with your target specifications and volume requirements:
📧 [email protected] | 💬 +86 15951276160 (WhatsApp)
Quote “CBCT-GUIDE-2026” for priority technical consultation and 2026 Q1 pricing lock
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: CBCT Scanner Procurement – Key FAQs for 2026
Frequently Asked Questions: CBCT Scanner Acquisition in 2026
As dental imaging technology advances, Cone Beam Computed Tomography (CBCT) systems remain a critical investment for comprehensive diagnostics. Below are five essential questions and expert answers addressing voltage compatibility, spare parts availability, installation protocols, and warranty terms—key considerations for clinics and distributors in 2026.
| Question | Answer | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. What voltage requirements should I verify before purchasing a CBCT scanner in 2026? |
Most modern CBCT scanners operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In North America, 120V systems are standard, while Europe, Asia, and the Middle East typically require 230V.
Key considerations: Pro Tip for Distributors: Stock models with auto-switching power supplies or offer region-specific configurations to streamline deployment. |
|||||||||||||||
| 2. How accessible are spare parts for CBCT scanners, and what components are most commonly replaced? |
Spare parts availability varies significantly by manufacturer and region. In 2026, leading OEMs (e.g., Carestream, Planmeca, KaVo Imaging) maintain global logistics networks, but lead times for high-demand components can range from 5 to 15 business days.
Commonly replaced parts include: Recommendation: Prioritize vendors offering local warehousing or distributor-held inventory agreements. For clinics, consider purchasing extended service contracts that include priority parts access. |
|||||||||||||||
| 3. What does the CBCT installation process involve, and who is responsible for it? |
Installation of a CBCT scanner is a multi-phase process typically managed by the manufacturer or certified third-party engineers. Responsibilities are often shared:
Manufacturer/Distributor Responsibilities: Clinic Responsibilities: Note: In 2026, modular and AI-assisted calibration tools are reducing installation time to 1–2 days for most mid-range units. |
|||||||||||||||
| 4. What warranty coverage should I expect with a new CBCT scanner in 2026? |
Standard warranties for new CBCT systems in 2026 typically include:
Best Practice: Always confirm whether the warranty is on-site or return-to-factory, and whether labor and travel are included. Distributors should negotiate service-level agreements (SLAs) with response times under 48 hours. |
|||||||||||||||
| 5. Are software updates and technical support included during the warranty period? |
In 2026, most premium CBCT manufacturers include remote software updates and basic technical support for the duration of the standard warranty (typically 2 years). Updates often deliver: • Enhanced image reconstruction algorithms • AI-powered diagnostic tools (e.g., automated implant planning, pathology detection) • Regulatory compliance patches (e.g., GDPR, HIPAA) Important: Post-warranty, software updates may require an annual subscription. Verify whether the system supports over-the-air (OTA) updates or requires on-site intervention. Distributor Advantage: Offer bundled support packages that include ongoing software access and remote diagnostics to increase customer retention. |
Need a Quote for Cbct Scan Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


