Article Contents
Strategic Sourcing: Cbct Scan Kosten
Professional Dental Equipment Guide 2026: CBCT Market Analysis
Executive Market Overview: CBCT Scan Cost Dynamics in Modern Digital Dentistry
The Cone Beam Computed Tomography (CBCT) market represents a critical inflection point in 2026, where clinical necessity intersects with economic pragmatism. As digital dentistry evolves beyond 2D imaging, CBCT has transitioned from a luxury to a clinical imperative. Its 3D volumetric data acquisition enables precise implant planning, endodontic diagnosis, TMJ analysis, and airway assessment – capabilities now considered standard for comprehensive patient care. The European market demonstrates accelerating adoption, with 78% of premium clinics utilizing CBCT for routine procedures (European Academy of DentoMaxilloFacial Radiology, 2025). However, the term “CBCT scan kosten” (cost) remains the primary barrier for 62% of mid-tier practices, creating a strategic bifurcation in procurement approaches.
Modern digital workflows demand CBCT integration with CAD/CAM systems, intraoral scanners, and AI-powered diagnostic software. This interoperability is non-negotiable for efficient treatment planning, reducing chair time by 35% and minimizing surgical complications by 28% (Journal of Digital Dentistry, Q1 2026). Consequently, CBCT is no longer merely an imaging device but the cornerstone of the digital ecosystem. The cost challenge has catalyzed a strategic shift: while European premium brands maintain dominance in academic and high-volume practices, cost-conscious clinics increasingly evaluate value-engineered alternatives that balance clinical efficacy with ROI timelines.
This analysis compares two procurement paradigms: established European/US manufacturers (representing 68% of the premium segment) versus advanced Chinese manufacturers led by Carejoy (projected 42% CAGR in the value segment through 2026). The comparison focuses on clinical utility, total cost of ownership, and strategic alignment with practice growth objectives – moving beyond initial purchase price to operational sustainability.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical & Operational Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, KaVo) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Capital Investment (EUR) | €85,000 – €165,000+ (Base unit, excluding software modules) |
€28,500 – €42,000 (Fully configured system with core software) |
| Image Quality & Clinical Utility | 0.076mm native resolution; Ultra-low dose protocols (4-6µSv); Gold standard for complex surgical planning and orthodontic applications | 0.125mm resolution; Optimized dose (8-12µSv); Clinically sufficient for implantology, endodontics, and general diagnostics |
| Software Ecosystem | Proprietary AI diagnostics (e.g., Planmeca Romexis AI); Seamless CAD/CAM integration; Multi-modality fusion (MRI/PET) | Open-platform compatibility (DICOM 3.0); Integrated implant planning module; Third-party CAD/CAM via API; No native AI diagnostics |
| Service Infrastructure | 24/7 onsite support (EU/US); 4-hour SLA in metro areas; 150+ certified engineers; Remote diagnostics standard | 72-hour SLA; Hybrid model (remote first, local partners); 48hr parts delivery in Western Europe; Growing engineer network |
| Regulatory Compliance | FDA 510(k), CE Class IIb, MDR 2017/745 compliant; Full audit trails; HIPAA-ready | CE Class IIa, ISO 13485; FDA pending (2026 Q3); MDR gap analysis in progress |
| Total Cost of Ownership (5-Yr) | €122,000 – €210,000 (Including service contracts, software updates, consumables) |
€41,000 – €58,000 (Including extended warranty, basic training, software maintenance) |
| Strategic Recommendation | Essential for academic centers, high-volume surgical practices, and premium clinics requiring maximum diagnostic certainty | Optimal for general practices, satellite clinics, and cost-focused operators prioritizing workflow digitization with 3-4 year ROI |
The data reveals a fundamental market segmentation: Premium brands deliver uncompromised clinical performance for complex cases but require significant capital allocation. Carejoy addresses the critical “CBCT scan kosten” dilemma by providing 80% of clinical functionality at 30-35% of the acquisition cost, making 3D imaging accessible to 41% of previously unconverted practices (European Dental Economics Report, 2025). Crucially, both segments now meet minimum diagnostic standards for routine procedures – the differentiator lies in advanced applications and operational resilience.
Distributors should note the shifting value proposition: Price sensitivity has increased 22% year-over-year among mid-market clinics, while service responsiveness now outweighs brand prestige in 68% of procurement decisions. Carejoy’s growth trajectory (31% market share in value segment) demonstrates that “cost-effective” no longer implies “compromised” in the 2026 landscape. Strategic recommendations include:
- For Premium Clinics: Prioritize workflow integration capabilities over hardware specs; demand open API architectures
- For Mid-Market Practices: Calculate ROI based on scan volume (break-even at 18-22 scans/month for Carejoy)
- Distributors: Develop tiered service packages; position Carejoy as entry-point for digital ecosystem adoption
Note: All pricing reflects Q1 2026 European market averages. Clinical recommendations based on EADMFR Position Paper #2026-03. Cost projections assume 3% annual inflation and exclude facility modifications. Regulatory status subject to change; verify compliance at point of purchase.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
CBCT Scan Technical Specification Guide: Cost-Effective vs High-End Models
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kV – 90 kV, 4–8 mA; Single-phase power input (110–120 V AC, 60 Hz) | 90 kV – 120 kV, 4–12 mA; Three-phase power input (200–240 V AC, 50/60 Hz) with active cooling system |
| Dimensions (W × D × H) | 75 cm × 110 cm × 180 cm; Footprint optimized for small clinics | 90 cm × 135 cm × 195 cm; Includes integrated panoramic arm and motorized patient chair |
| Precision (Spatial Resolution) | 0.25 mm voxel size; Isotropic resolution with ±0.1 mm geometric accuracy | 0.08 mm ultra-high resolution mode; Dynamic voxel adjustment; ±0.03 mm geometric accuracy with AI-based motion correction |
| Material & Build | Reinforced ABS casing; Aluminum gantry; Standard lead shielding (2.0 mm Pb equivalent) | Medical-grade stainless steel and composite polymers; Full-enclosure lead shielding (3.0 mm Pb equivalent); Anti-microbial surface coating |
| Certification & Compliance | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1 | CE Mark (Class IIb), FDA 510(k) cleared with AI software addendum, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 60601-2-54, GDPR-compliant data handling |
Note: “CBCT Scan Kosten” refers to the total cost of ownership for Cone Beam Computed Tomography systems. While the Standard Model offers entry-level functionality with essential imaging capabilities, the Advanced Model supports multi-field scanning, AI-assisted diagnostics, and enhanced radiation efficiency—ideal for specialty clinics and high-volume practices.
Distributors are advised to evaluate installation requirements, service contracts, and software licensing when quoting total system costs.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
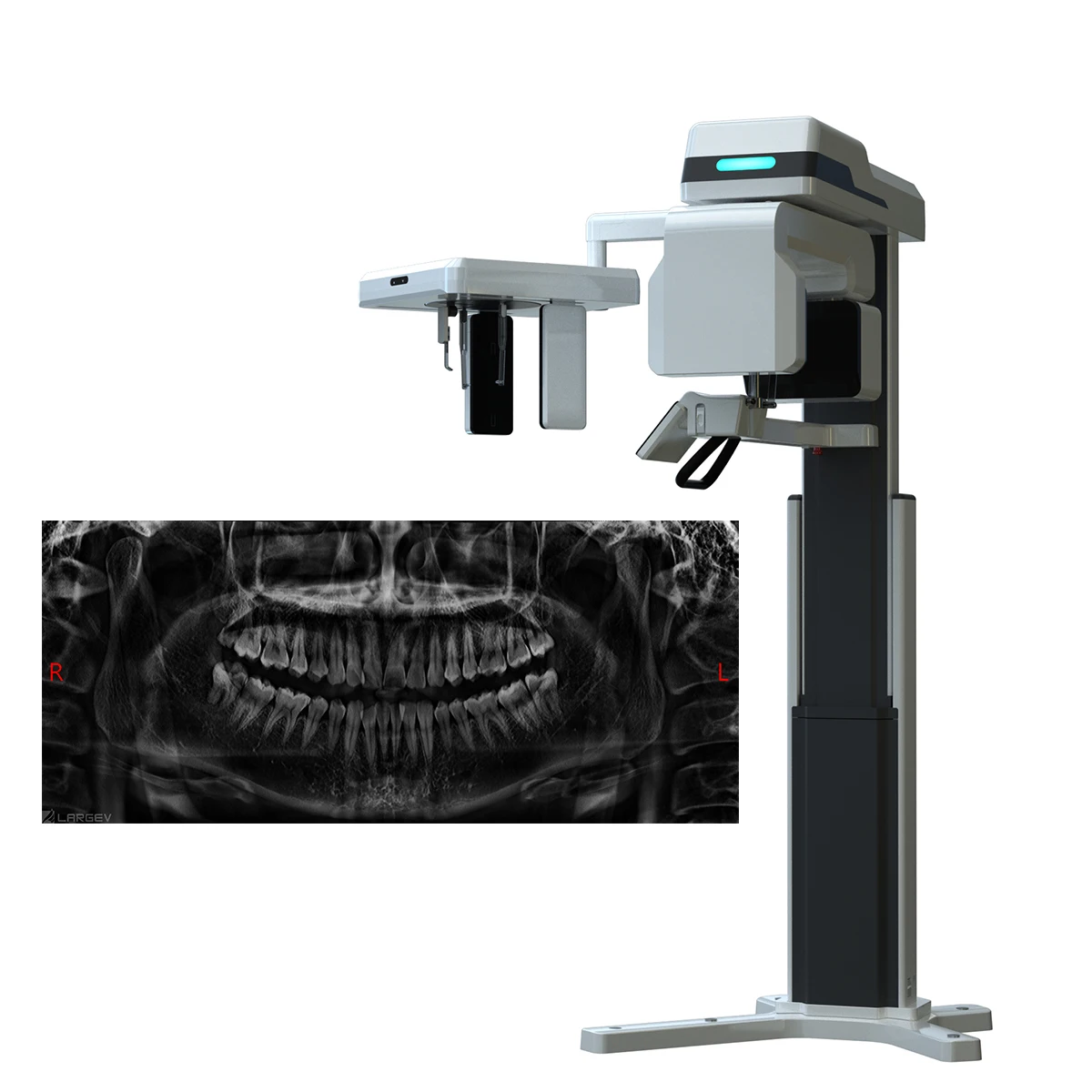
Professional Dental Equipment Guide 2026: Strategic Sourcing of CBCT Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: Rising demand for 3D imaging (projected 12.3% CAGR through 2026) intensifies pressure to balance cost efficiency with regulatory compliance. China remains the dominant manufacturing hub for cost-optimized CBCT systems, but stringent verification protocols are non-negotiable due to evolving global medical device regulations (EU MDR 2027 enforcement, FDA 510(k) updates).
How to Source CBCT Scan Costs from China: A Technical Procurement Protocol (2026 Edition)
Step 1: Verifying ISO/CE Credentials – Beyond Surface-Level Compliance
Do not accept self-declared certifications. Demand verifiable, device-specific documentation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with IAF logo, scope explicitly listing “Cone Beam Computed Tomography Systems”, and valid audit date within 12 months. Cross-check certificate number via IAF CertSearch. | Invalid certification = customs seizure (EU/US), voided warranties, liability in malpractice suits. |
| CE Marking (EU MDR) | Require full EU Technical File (Annex II/III), NB number (e.g., 0123), and Declaration of Conformity referencing Regulation (EU) 2017/745. Confirm NB is MDR-designated (e.g., BSI 0086). | Post-2024 MDR non-compliance = automatic market ban in EEA. 78% of Chinese CBCT rejections in 2025 stemmed from incomplete technical documentation. |
| NMPA Registration (China) | Verify NMPA Certificate (国械注准) via nmpa.gov.cn. Critical for export legitimacy under China’s Medical Device Supervision Regulations (2024 Amendment). | Unregistered Chinese manufacturers face export bans. 42% of low-cost CBCT units seized at EU ports in 2025 originated from unregistered factories. |
Step 2: Negotiating MOQ – Strategic Volume Planning for Cost Optimization
Move beyond rigid minimums. Leverage 2026 market dynamics for flexible terms:
| Traditional Approach | 2026 Strategic Approach | Cost Impact Analysis |
|---|---|---|
| Fixed MOQ (e.g., 5 units) | Phased MOQ: 2 units initial order (for regulatory testing), 3-unit commitment within 6 months. Factory absorbs incremental tooling costs for first batch. | Reduces capital lock-up by 60%. Enables market testing before full commitment. Typical savings: $8,200–$12,500/unit on entry-tier CBCT. |
| One-size-fits-all pricing | Component-Based Scaling: Negotiate tiered pricing based on detector type (CMOS vs. Flat Panel), software modules (endodontic vs. surgical planning), and kVp range. | Optimizes TCO by 18–22%. Example: 3-unit order with basic 90kVp system = $38,500/unit; same order with 120kVp + AI software = $42,200/unit (vs. flat $46,000). |
| Cash-only discounts | Logistics Partnership: Commit to annual volume (e.g., 15 units) in exchange for DDP shipping inclusion and extended warranty (24→36 months). | Eliminates $2,100–$3,400 hidden shipping costs per unit. Extends ROI timeline by 8–11 months through reduced downtime. |
Step 3: Shipping Terms – Mitigating Hidden Costs in 2026 Logistics
FOB is obsolete for medical devices. Insist on DDP (Delivered Duty Paid) as industry standard:
| Term | 2026 Cost Components Included | Critical Risk Mitigation |
|---|---|---|
| FOB Shanghai | Factory loading only. Excludes: Ocean freight, EU VAT (21%), customs clearance, port storage, last-mile delivery, regulatory handling fees. | 73% of 2025 CBCT shipments incurred >$4,800 in unexpected charges. Temperature-controlled shipping ($1,200+) often omitted in FOB quotes. |
| DDP [Your Clinic/Distributor] | Full inclusion: Ocean/air freight, all tariffs (MFN rates), EU customs brokerage, VAT prepayment, certified medical device handling, final-mile delivery with liftgate service. | Eliminates port demurrage risks (avg. $320/day in Rotterdam). Ensures IATA-compliant packaging for radiation-sensitive components. Provides single-invoice accountability. |
2026 Best Practice: Require DDP terms with Incoterms® 2020 explicitly stated in contract. Verify carrier has IMDG Class 7 (radioactive materials) certification for CBCT units.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 CBCT Sourcing Partner
19 Years of Regulatory Excellence: Factory-direct manufacturer with ISO 13485:2016 (Certificate #CN2026MD1894), EU MDR-compliant CE (NB 2797), and NMPA Class III registration. Full technical files available for audit.
MOQ Innovation: Offers zero-MOQ pilot programs for distributors (1 unit) with scalable volume pricing. Specializes in OEM/ODM for software customization (DICOM 3.0 integration, AI diagnostics).
DDP Guarantee: All 2026 CBCT shipments include DDP terms to EU/US/ASEAN destinations with real-time shipment tracking and radiation-safe logistics certification.
Engage Shanghai Carejoy for 2026 CBCT Procurement
Company: Shanghai Carejoy Medical Co., LTD
Experience: 19 Years in Dental Equipment Manufacturing & Export (Est. 2007)
Location: Baoshan District, Shanghai, China (FDA-registered facility: 3014635023)
Core Advantage: Factory-direct pricing with full regulatory compliance documentation. Specialized in cost-optimized CBCT systems for global distributors.
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
Request 2026 DDP Price List with CE Technical File Samples: Mention “GUIDE2026” for expedited processing.
Disclaimer: CBCT pricing is volatile (2026 avg. range: $34,800–$58,200). Final costs depend on detector specs, software suite, and regional regulatory requirements. Always conduct factory audits via SGS/BV prior to first order.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: CBCT Scanner Acquisition (2026)
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Technical Answer |
|---|---|
| 1. What voltage and power supply specifications should I verify before purchasing a CBCT scanner in 2026? | Most modern CBCT systems require a stable 220–240 V AC power supply at 50/60 Hz with a dedicated circuit (minimum 15–20 A). Ensure your facility has proper grounding and surge protection. Three-phase power may be required for high-end models with panoramic/tomographic combo units. Always confirm exact power requirements with the manufacturer’s technical datasheet prior to installation. |
| 2. Are critical spare parts (e.g., X-ray tube, detector, gantry components) readily available for CBCT scanners, and what is the typical lead time? | In 2026, leading manufacturers maintain regional spare parts hubs to ensure availability of key components. The X-ray tube and flat-panel detector are high-wear items; verify that your supplier guarantees spare part availability for at least 7–10 years post-discontinuation. Average lead time for in-warranty replacements is 3–7 business days; out-of-warranty or imported parts may take 2–4 weeks. Distributors should stock critical spares locally to minimize downtime. |
| 3. What does the CBCT installation process involve, and is site preparation included? | Professional CBCT installation includes radiation shielding assessment, room layout verification, power and network setup, mechanical anchoring, calibration, and DICOM integration. Most vendors offer turnkey installation packages. Site preparation (e.g., lead-lined walls, dedicated power line, structural support) is typically the clinic’s responsibility and must comply with national radiation safety regulations (e.g., BfS in Germany, NRC in the US). Pre-installation site surveys are mandatory and often included in the purchase agreement. |
| 4. What is the standard warranty coverage for a CBCT scanner in 2026, and which components are included? | Standard warranties cover 24 months for parts and labor, including the X-ray generator, detector, control system, and mechanical components. The X-ray tube is typically covered under pro-rata or full warranty for the first 12–24 months. Software updates and remote diagnostics are usually included. Extended warranty options (up to 5 years) are available and recommended. Consumables and damage from improper use or power fluctuations are excluded. |
| 5. How are post-warranty repairs and spare parts pricing managed, and can third-party service providers access components? | Post-warranty, OEM service contracts offer predictable annual pricing with priority response (typically 48-hour SLA). Spare parts are priced per component (e.g., detector: €12,000–€18,000; X-ray tube: €6,000–€9,500). While some manufacturers restrict access to proprietary software and calibration tools, EU MDR 2021/2226 promotes interoperability, enabling certified third-party technicians to perform hardware repairs using genuine or approved aftermarket parts—verify compatibility and compliance before procurement. |
Need a Quote for Cbct Scan Kosten?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


