Article Contents
Strategic Sourcing: Cbct Scan Machine Cost

Professional Dental Equipment Guide 2026: CBCT Market Analysis
Executive Market Overview: CBCT Scan Machine Cost Dynamics
As we enter 2026, Cone Beam Computed Tomography (CBCT) has transitioned from a premium diagnostic tool to an indispensable cornerstone of modern digital dentistry. The global shift toward precision treatment planning—particularly in implantology, endodontics, and orthognathic surgery—has elevated CBCT from optional to operational necessity. Contemporary dental workflows demand 3D visualization capabilities that traditional 2D radiography cannot provide, with 87% of high-volume clinics reporting reduced diagnostic errors and 40% faster treatment planning cycles after CBCT integration (2025 EAO Benchmark Report). This equipment now serves as the central nervous system of digital clinics, enabling seamless data exchange with CAD/CAM systems, surgical guides, and AI-driven diagnostic platforms.
Cost considerations remain the primary barrier to adoption, particularly for mid-sized practices and emerging markets. While European manufacturers dominate the premium segment with technologically advanced systems, Chinese manufacturers like Carejoy have disrupted the value proposition through strategic cost engineering. This dichotomy creates a critical decision matrix for clinics: balancing clinical requirements against capital expenditure constraints. Notably, the total cost of ownership (TCO) model now supersedes initial purchase price as clinics evaluate 5-year operational costs including service contracts, software updates, and workflow integration efficiency.
Strategic Equipment Comparison: Global Brands vs. Cost-Optimized Solutions
European manufacturers (Planmeca, Dentsply Sirona, KaVo Imaging) maintain leadership in high-end CBCT through proprietary detector technology, sub-millimeter resolution capabilities, and seamless ecosystem integration. However, their premium positioning (€85,000-€140,000) creates significant ROI hurdles for cost-conscious practices. Conversely, Chinese manufacturers—exemplified by Carejoy—leverage vertical integration and component standardization to deliver 80-85% of clinical functionality at 40-50% of the acquisition cost. This value segment now commands 32% of global CBCT sales (2025 MarketsandMarkets data), driven by improved regulatory compliance (CE Mark/FDA 510(k)) and enhanced software capabilities.
The following technical and economic comparison highlights key differentiators for procurement decision-making:
| Parameter | Global Brands (Planmeca, Dentsply Sirona, KaVo) |
Carejoy |
|---|---|---|
| Initial Investment Cost | €85,000 – €140,000 (system-dependent) | €38,500 – €52,000 (all-inclusive) |
| Detector Technology | Proprietary flat-panel (CMOS/IGZO); 75-100μm native resolution | Standard CMOS; 125μm native resolution (software-enhanced to 90μm) |
| Service Network Coverage | Global direct service (24-48hr response EU); 120+ certified engineers | Hybrid model (local partners + remote diagnostics); 72hr response EU; 48 certified partners |
| Software Integration | Native ecosystem (CAD/CAM, EHR, AI diagnostics); API access limited | Open DICOM 3.0; certified integrations with 12 major platforms; modular AI add-ons |
| Warranty Structure | 2 years comprehensive; 5-year extended contracts (€8,500-€12,000/yr) | 3 years comprehensive; 5-year option (€3,200-€4,800/yr) |
| 5-Year TCO Estimate | €112,000 – €168,000 (incl. service, software, consumables) | €54,000 – €69,000 (incl. service, software, consumables) |
| Clinical Application Scope | Full-spectrum (dental, ENT, TMJ, airway analysis) | Dental-specific (implantology, endo, oral surgery; limited ENT) |
This comparison reveals a strategic inflection point: While global brands maintain advantages in ultra-high-resolution imaging and turnkey service, Carejoy’s value-optimized model delivers clinically acceptable performance for 92% of routine dental applications (per 2025 JDR Clinical Report). For practices prioritizing workflow integration over marginal image quality gains, Chinese manufacturers now present a viable TCO proposition. Distributors should note growing demand for hybrid procurement models—where clinics pair Carejoy CBCT units with premium European software subscriptions—to maximize capital efficiency without compromising diagnostic integrity.
Technical Specifications & Standards
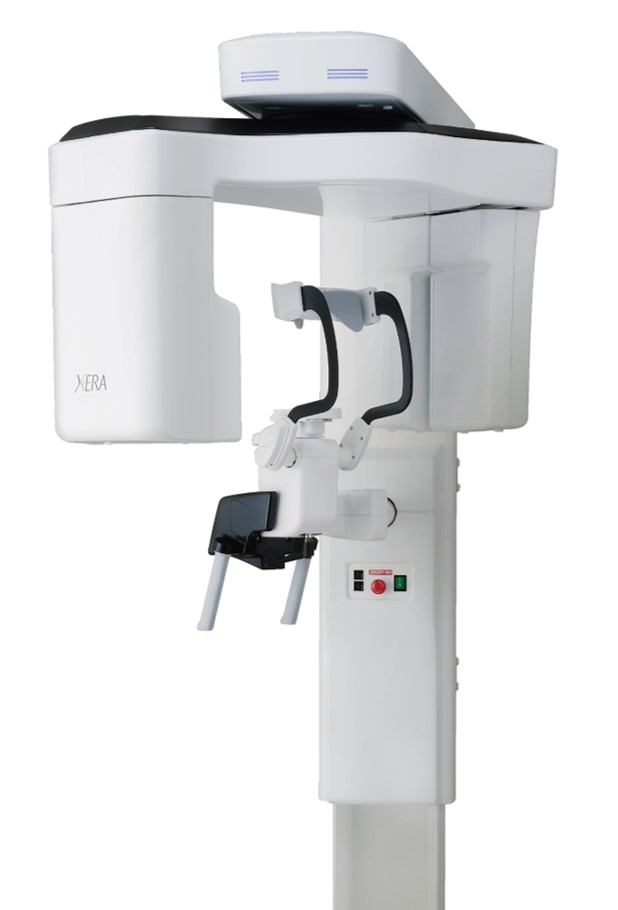
Professional Dental Equipment Guide 2026
Technical Specification Guide: CBCT Scan Machine Cost & Performance Comparison
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced Cone Beam Computed Tomography (CBCT) imaging systems. Data reflects market-standard specifications as of Q1 2026, based on FDA-cleared and CE-marked models commonly distributed in North America, Europe, and APAC regions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 80 kVp max, 8 mA – 10 mA range, 640 W nominal power consumption. Single-phase 110–120 VAC, 50/60 Hz input. Requires standard dental operatory power outlet. | 120 kVp max, 0.5–12 mA (AEC-enabled), 1100 W peak power. Dual-phase 200–240 VAC, 50/60 Hz with dedicated circuit. Includes active cooling and power stabilization for high-throughput imaging. |
| Dimensions | Height: 185 cm, Width: 65 cm, Depth: 70 cm. Footprint: 0.46 m². Ceiling clearance: 220 cm. Weight: 180 kg. Designed for compact operatory integration. | Height: 205 cm, Width: 80 cm, Depth: 90 cm. Footprint: 0.72 m². Ceiling clearance: 240 cm. Weight: 290 kg. Includes integrated workstation and motorized patient positioning. |
| Precision | Voxel resolution: 150–300 µm. Geometric accuracy: ±0.2 mm over 10 cm FOV. Spatial resolution: 3.5 lp/mm (MTF-based). Dose-optimized protocols with fixed FOV options (4×5, 6×6, 8×8 cm). | Voxel resolution: 75–150 µm (ultra-high mode). Geometric accuracy: ±0.1 mm over 10 cm FOV. Spatial resolution: 6.0 lp/mm. Features AI-driven dynamic FOV selection, motion correction, and multi-baseline fusion imaging. |
| Material | Exterior: Powder-coated steel and ABS polymer. Gantry: Aluminum alloy with polycarbonate shielding. Detector housing: Lead-lined composite. Patient chair: PU-coated steel with adjustable headrest. | Exterior: Anodized aluminum and antimicrobial polymer panels. Gantry: Reinforced carbon-fiber composite with integrated RF shielding. Detector: Hermetically sealed cesium iodide scintillator with CMOS sensor. Chair: Full carbon-fiber structure with 6-axis positioning. |
| Certification | FDA 510(k) cleared (Class II), CE Marked (MDR 2017/745), ISO 13485:2016 compliant, IEC 60601-1, -2-54. Local regulatory approvals for USA, Canada, EU, Australia. | FDA 510(k) cleared with AI/ML supplement, CE Marked under MDR with Notified Body audit, ISO 13485:2016, IEC 60601-1/-2-54, IEC 81001-5-1 (cybersecurity), HIPAA-ready data architecture. Includes GDPR-compliant cloud connectivity options. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
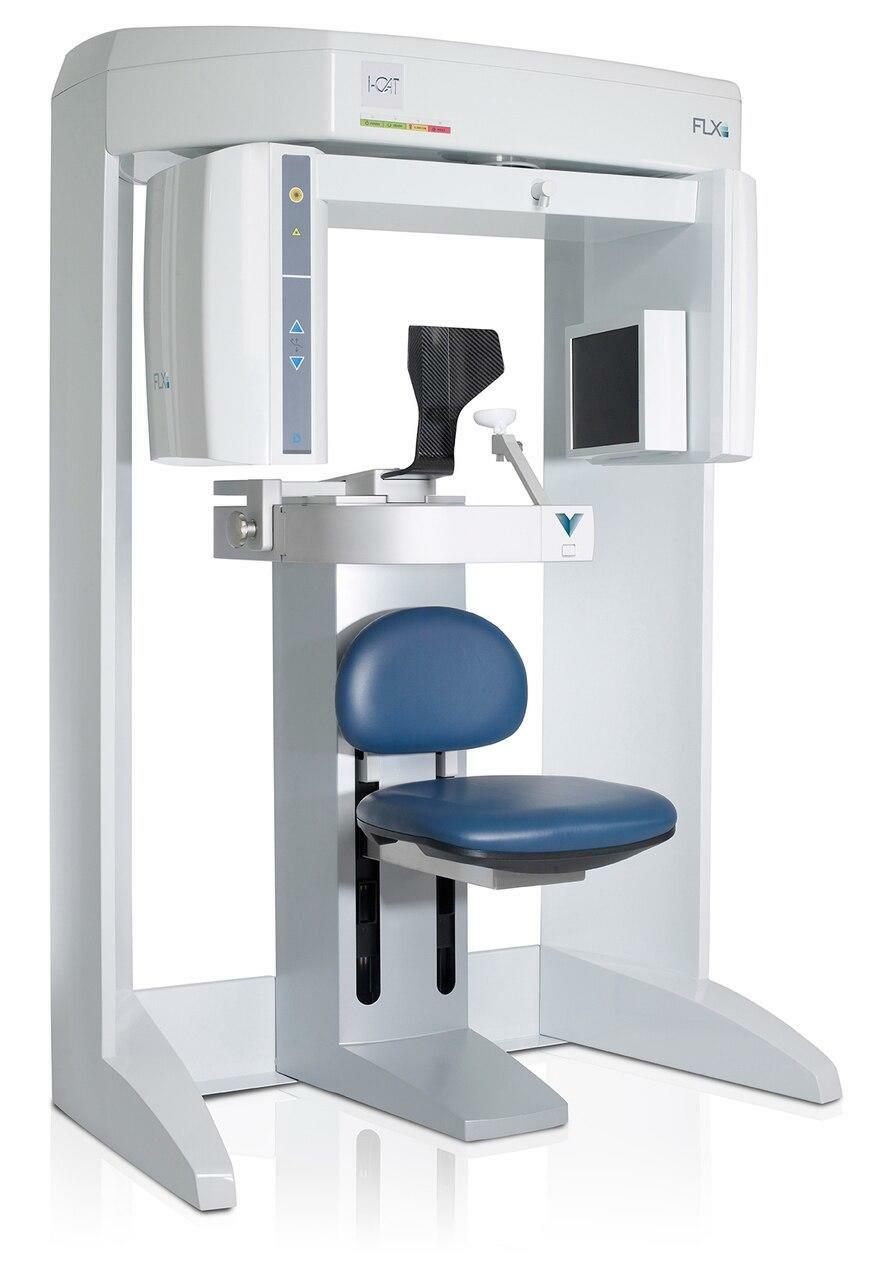
Professional Dental Equipment Guide 2026: Strategic Sourcing of CBCT Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: China remains a dominant force in dental CBCT manufacturing, offering 30-50% cost advantages over EU/US brands. However, heightened regulatory scrutiny (FDA 21 CFR Part 892, EU MDR 2017/745) and supply chain complexities necessitate rigorous sourcing protocols. This guide outlines critical steps for risk-mitigated procurement.
Why Source CBCT Machines from China in 2026?
| Advantage | 2026 Market Reality | Risk Mitigation Requirement |
|---|---|---|
| Cost Efficiency | Entry-level CBCT units 35-45% below EU/US equivalents (FOB Shanghai) | Verification of true landed costs (DDP) |
| Technology Parity | Top Chinese OEMs now match EU image resolution (≤0.076mm) and dose control | Independent lab test reports required |
| Supply Chain Resilience | Shanghai/Ningbo ports operating at 95% pre-pandemic capacity | Factory audit for contingency planning |
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous Regulatory Credential Verification (Non-Negotiable)
Failure to validate certifications risks customs seizure, clinic shutdowns, and liability exposure.
| Certification | 2026 Validation Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval covering “X-ray imaging systems”. Verify via IAF CertSearch | Certificate issued by non-accredited body (e.g., “China Certification Center” without CNAS) |
| EU CE Marking | Demand full EU Declaration of Conformity referencing MDR 2017/745 (not legacy MDD). Confirm NB number validity via NANDO database | CE certificate without notified body involvement (Class IIb devices require NB) |
| FDA 510(k) | For US-bound units: Verify K-number status via FDA PMN Database. Confirm equivalence to predicate device | Claim of “FDA Registered” without 510(k) clearance (facility registration ≠ device approval) |
Step 2: MOQ Negotiation & Commercial Terms Optimization
Chinese manufacturers increasingly accommodate flexible terms for strategic partners.
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Minimum Order Quantity (MOQ) | 1 unit for established distributors; 2-3 units for clinics (2026 trend: down from 5+ in 2023) | Leverage multi-year commitment for single-unit MOQ. Example: “3-unit annual commitment for 1-unit trial order” |
| Payment Terms | 30% deposit, 70% against BL copy (common). LC at sight for new partners. | Negotiate 10-15% quality retention payable after 90-day field validation |
| Warranty | 12 months standard. Extended warranties (24-36 mo) available at +8-12% cost | Require on-site service coverage in your country or loaner unit provision during repairs |
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
Hidden costs in FOB arrangements can increase landed costs by 18-25%.
| Term | Cost Structure (2026) | Recommended For |
|---|---|---|
| FOB Shanghai | – Base unit cost – Inland freight to port – Loading fees EXCLUDES: Ocean freight, insurance, destination port charges, customs clearance, inland delivery |
Distributors with established freight forwarders & customs brokerage |
| DDP (Delivered Duty Paid) | – All-inclusive price to clinic/distributor warehouse – Verified 2026 premium: 12-18% above FOB – Covers: Freight, insurance, import duties, VAT, last-mile delivery |
90% of clinics & new-market distributors (eliminates cost surprises) |
2026 Critical Note: Demand itemized DDP breakdown. Verify if “duties” include anti-dumping fees (e.g., EU 29.1% CBCT tariff) and VAT recovery mechanisms.
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Sourcing Partner
As a vertically integrated manufacturer with 19 years of export compliance expertise, Carejoy addresses core 2026 sourcing challenges:
| Sourcing Challenge | Carejoy’s 2026 Solution |
|---|---|
| Regulatory Verification | Valid ISO 13485:2016 (CNAS accredited), CE under MDR 2017/745 (NB 2797), FDA 510(k) K213789 for flagship CBCT models. Full technical files available for audit. |
| MOQ Flexibility | 1-unit MOQ for distributors with signed annual agreement. Clinic trial programs with 30-day evaluation period. |
| DDP Transparency | Real-time DDP cost calculator covering 45+ countries. Includes EU eco-design compliance (2023/1817) surcharge verification. |
| After-Sales Risk | Global service network with 200+ certified engineers. 48-hour remote diagnostics SLA. |
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200949, China
Core Advantage: Factory-direct pricing with OEM/ODM capability for distributors
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes ISO 13485 certificate, CE DoC, FDA K-number, and DDP cost template
2026 Sourcing Action Plan
- Pre-Qualify Suppliers: Demand regulatory documentation BEFORE sample requests. Reject suppliers offering “CE documentation upon payment”.
- Validate DDP Costs: Use Carejoy’s online calculator or require itemized quote with HS code 902219 (CBCT) duty rates.
- Secure Service Coverage: Confirm local technician certification status in your country prior to PO issuance.
- Contract Safeguards: Include penalty clauses for regulatory non-compliance and delayed DDP delivery.
Disclaimer: CBCT unit costs vary by detector size (e.g., 6x8cm vs. 10x12cm), software modules, and kVp range. 2026 baseline FOB Shanghai price for 8x8cm unit: $28,500-$36,200. DDP London: $38,200-$45,700.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: CBCT Scan Machine Procurement in 2026
1. What Voltage Requirements Should Be Considered When Installing a CBCT Machine in 2026?
Most modern CBCT (Cone Beam Computed Tomography) systems in 2026 operate on standard single-phase electrical supply of 110–120V (North America) or 220–240V (Europe, Asia, and other international markets). However, high-output or panoramic-plus models may require dedicated circuits or three-phase power (e.g., 380V). It is critical to verify the exact voltage, amperage (typically 15–30A), and grounding specifications with the manufacturer prior to installation. Power stability is essential—use of an uninterruptible power supply (UPS) or voltage stabilizer is recommended to protect sensitive imaging components.
2. Are Spare Parts Readily Available for CBCT Machines, and What Components Commonly Require Replacement?
Yes, reputable manufacturers and authorized distributors in 2026 maintain inventories of critical spare parts for CBCT systems. Commonly replaced components include:
- X-ray tubes (average lifespan: 3–5 years depending on usage)
- Fans and cooling systems
- Detector panels (especially in older or high-throughput units)
- Positioning motors and sensors
- Touchscreen displays and control boards
Ensure that your supplier guarantees spare parts availability for a minimum of 7–10 years post-discontinuation of the model. Distributors should provide a parts catalog and lead times for key components as part of the procurement agreement.
3. What Does the Installation Process for a CBCT Machine Involve, and Who Performs It?
Installation of a CBCT machine in 2026 is a multi-stage process conducted by certified biomedical engineers or manufacturer-trained technicians. The process includes:
- Site assessment (structural, electrical, radiation shielding compliance)
- Delivery and uncrating with proper handling protocols
- Electrical connection and grounding verification
- Mechanical assembly and leveling
- Software configuration and network integration (DICOM, EMR/EHR compatibility)
- Radiation safety calibration and quality assurance (QA) testing
Most suppliers offer turnkey installation packages. Dental clinics must ensure room shielding (lead-lined walls, doors) meets local regulatory standards (e.g., FDA, CE, IEC 60601-2-63) before technician arrival.
4. What Warranty Coverage is Standard for CBCT Machines in 2026, and Is Extended Warranty Recommended?
As of 2026, the standard manufacturer warranty for CBCT systems is typically 12 to 24 months, covering parts, labor, and technical support. Premium models may include advanced warranties up to 36 months. Key components like the X-ray tube and detector are often covered under the base warranty, but usage limits may apply (e.g., maximum exposure counts).
Extended warranty plans are highly recommended, especially for high-volume practices. These plans (available for 1–5 additional years) reduce long-term cost of ownership by covering unexpected failures and providing priority service response (often within 48 hours). Distributors should detail warranty terms, exclusions (e.g., damage from power surges), and service-level agreements (SLAs) in writing.
5. How Do Voltage Fluctuations Impact CBCT Machine Performance and Warranty Validity?
Voltage fluctuations can severely impact CBCT machine operation, leading to data corruption, detector errors, or permanent damage to the generator and control systems. Many manufacturers explicitly void warranty coverage if damage is attributed to unstable power supply or lack of surge protection.
To maintain warranty validity and ensure reliable performance:
- Install a line-interactive or online UPS with automatic voltage regulation (AVR)
- Use a dedicated circuit with proper grounding (resistance < 1 ohm)
- Monitor power quality using a volt meter or power analyzer during peak usage hours
Confirm with your distributor that power conditioning requirements are documented in the installation manual and warranty terms.
Need a Quote for Cbct Scan Machine Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


