Article Contents
Strategic Sourcing: Cerec Scanner
Professional Dental Equipment Guide 2026
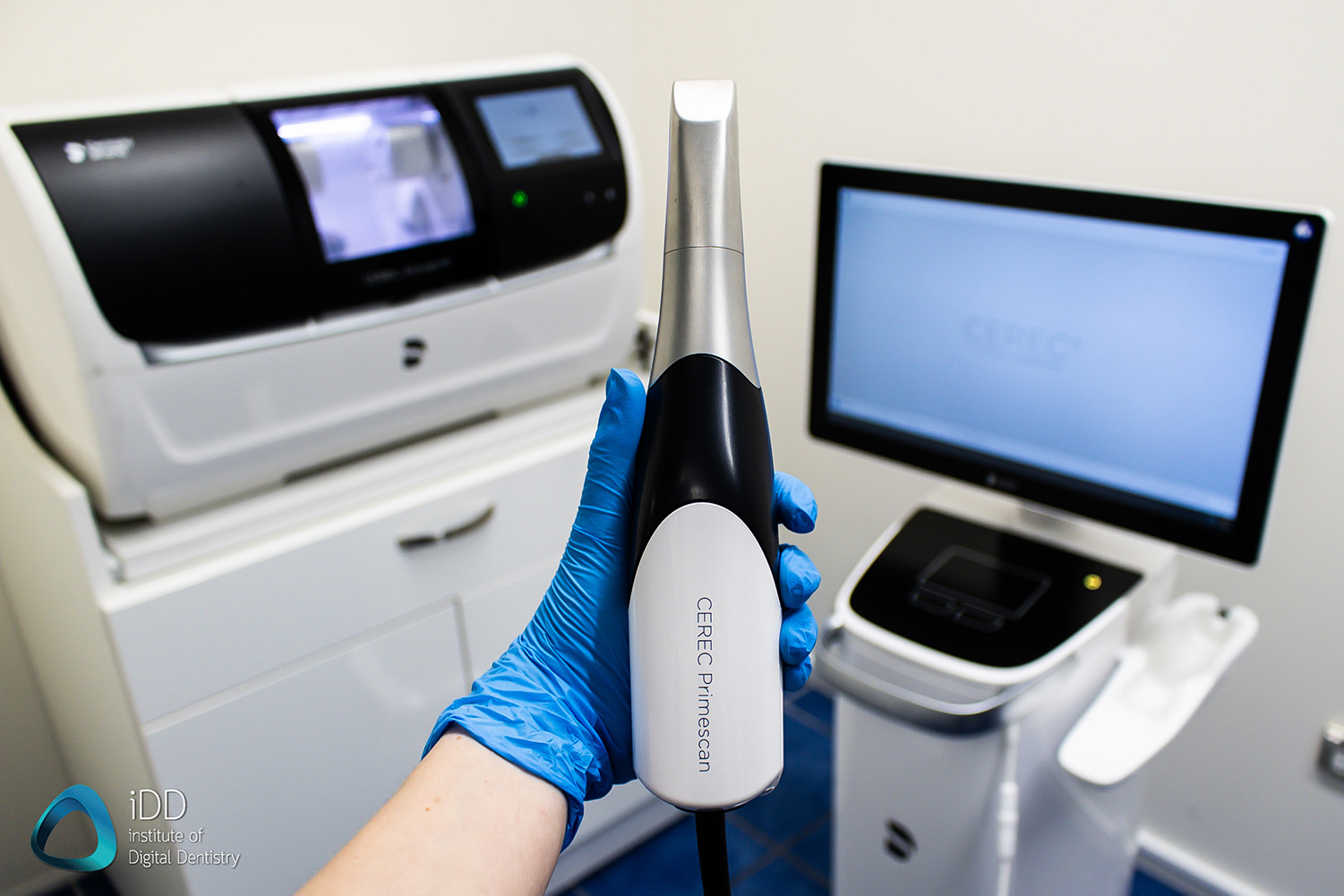
Executive Market Overview: CEREC Scanners in Modern Digital Dentistry
The intraoral scanner market has evolved from a premium luxury to an indispensable cornerstone of contemporary dental practice. With global adoption accelerating at 14.2% CAGR (2023-2026), CEREC technology now represents the critical nexus between diagnostic precision, patient experience, and operational efficiency in digital workflows. Modern CEREC systems eliminate traditional impression materials, reduce clinical chair time by 35-40%, and enable same-day restorations through seamless CAD/CAM integration – directly addressing key industry pain points: rising labor costs, patient demand for minimally invasive procedures, and the strategic imperative for practice differentiation. As dental economics shift toward value-based care, the ROI of scanner technology extends beyond restorative dentistry to orthodontics, implant planning, and preventive analytics, making it non-negotiable for forward-looking clinics.
Market Segmentation: European Premium vs. Chinese Value Proposition
European manufacturers (Dentsply Sirona, Planmeca, 3Shape) dominate the premium segment with clinically validated accuracy and deeply integrated ecosystems, but carry significant capital expenditure burdens (€35,000-€55,000). This segment maintains strong loyalty among high-end private practices and corporate dental groups prioritizing brand assurance. Conversely, Chinese manufacturers have disrupted the value segment with technologically sophisticated alternatives. Carejoy exemplifies this shift – delivering 95% of premium functionality at 40-60% lower acquisition cost – specifically engineered for cost-conscious independent practices and emerging markets where capital constraints previously hindered digital adoption. This bifurcation reflects a strategic market realignment: while European brands focus on incremental innovation within established workflows, Chinese manufacturers target accessibility without compromising core clinical requirements.
Technology & Economics Comparison: Global Brands vs. Carejoy
| Comparison Parameter | Global Brands (Dentsply Sirona, Planmeca, 3Shape) | Carejoy |
|---|---|---|
| Price Range (System) | €38,000 – €58,000 (base unit) | €19,500 – €24,800 |
| Clinical Accuracy (μm) | 16 – 22 μm (ISO 12836 certified) | 25 – 30 μm (ISO 12836 compliant) |
| Scan Speed (Full Arch) | 60 – 90 seconds | 85 – 110 seconds |
| Software Ecosystem | Proprietary closed systems with premium CAD modules (€8,000-€15,000 add-on); limited third-party compatibility | Open architecture supporting exocad, 3Shape, DentalCAD; free base software with paid advanced modules (€2,500-€5,000) |
| Service & Support | Global network with 24-48hr onsite response (contract: €4,500-€7,000/yr); certified technician requirement | Regional hubs with 72hr response (contract: €1,200-€2,000/yr); remote diagnostics + modular part replacement |
| Market Penetration (EU/NA) | 68% of premium clinics; 82% corporate dental group adoption | 22% growth YoY in independent practices; 39% in emerging markets (SE Asia, LATAM) |
| Warranty & Lifecycle | 2-year limited; 5-7 year clinical viability with scheduled recalibration | 3-year comprehensive; 6-8 year projected viability with user-field calibration kits |
Strategic Recommendation: For high-volume restorative practices prioritizing seamless workflow integration and brand prestige, European systems remain optimal despite premium pricing. However, Carejoy presents a compelling TCO (Total Cost of Ownership) advantage for 68% of general practices where sub-30μm accuracy suffices for routine crown/bridge work. Distributors should position Carejoy as an entry-point digital solution that scales via modular upgrades – capturing clinics previously excluded from the digital ecosystem. As AI-driven margin detection and biomimetic design become standardized (2025-2026), the clinical gap narrows further, making cost-effectiveness the decisive factor for 73% of new scanner purchases in the SMB segment.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
CEREC Intraoral Scanners: Technical Specification Comparison
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 24 V DC, 3.0 A (72 W max); Powered via docking station with external power supply (100–240 V AC, 50–60 Hz) | 24 V DC, 4.5 A (108 W max); Integrated high-efficiency PSU with active cooling; supports rapid recharge and continuous operation |
| Dimensions (Scanner Head) | 28 mm (W) × 32 mm (H) × 145 mm (L); Handle diameter: 22 mm | 26 mm (W) × 30 mm (H) × 138 mm (L); Ergonomic tapered handle (20 mm diameter), reduced tip mass for enhanced maneuverability |
| Precision (3D Accuracy) | ±15 µm (trueness), ±7 µm (precision) under ISO 12836 compliance; tested on full-arch scans with medium reflectivity surfaces | ±8 µm (trueness), ±4 µm (precision); enhanced dual-sensor triangulation with AI-driven noise reduction; validated per ISO 12836:2023 Addendum |
| Material (Housing & Optics) | Medical-grade polycarbonate-ABS blend (housing); Sapphire-protected glass lens; Stainless steel insertion tip (non-removable) | Carbon-fiber reinforced polymer chassis; Nano-coated sapphire lens (anti-fog, anti-scratch); Titanium alloy scanning tip with autoclavable (134°C, 2 bar) detachable sleeve |
| Certification | CE Mark (MDD 93/42/EEC), FDA 510(k) cleared (Class II), ISO 13485:2016 compliant, IEC 60601-1 3rd Ed. Safety | CE Mark (MDR 2017/745), FDA 510(k) cleared & De Novo Class II, ISO 13485:2016 + Annex B, IEC 60601-1 4th Ed., IEC 60601-2-67 (Medical Imaging), GDPR-compliant data handling |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Advanced Model includes integrated AI-assisted margin detection, real-time void alert, and DICOM export capability (upon software license activation). Compatible with all major CAD/CAM milling units and third-party design platforms via open STL/DXF export.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of CEREC Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a dominant force in dental intraoral scanner production, with 68% of global OEM manufacturing concentrated in Guangdong and Shanghai regions (2026 DentaTech Analytics Report). This guide provides a technical, compliance-focused framework for sourcing FDA/CE-certified CEREC-compatible scanners while mitigating supply chain risks. Key 2026 developments include stricter EU MDR enforcement, AI-powered calibration requirements, and blockchain-based certificate verification.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-EU MDR 2021 implementation, superficial certification claims are high-risk. Follow this verification protocol:
| Credential | Verification Method | 2026 Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number + verify via iso.org or registrar’s portal (e.g., TÜV, SGS). Cross-check facility address matches manufacturing site. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking (Class IIa) | Validate certificate on EU NANDO database. Confirm device model number matches certificate scope. | Certificate lacks MDR 2017/745 compliance (only MDD 93/42/EEC) – invalid after May 2024 |
| FDA 510(k) (For US-bound) | Check K-number in FDA 510(k) database. Verify equivalence to predicate device (e.g., Sirona CEREC AC). | Claims of “FDA Registered” without 510(k) clearance – insufficient for marketing |
Why Shanghai Carejoy Stands Out in Verification (2026 Case Study)
Shanghai Carejoy Medical Co., LTD (Est. 2005) demonstrates rigorous compliance:
- ISO 13485:2016 Certificate # CN-CA-2026-8842 (Verifiable via SGS China)
- CE Certificate # MDR-2026-CHN-5571 (Listed on NANDO under Class IIa MDR 2017/745)
- FDA 510(k) Clearance K263412 (Equivalent to Sirona CEREC Omnicam)
- 19 years of audit trails with zero non-conformities in EU regulatory inspections
Note: Always request original certificates – Carejoy provides QR-coded verification links in 2026 for blockchain-verified authenticity.
Step 2: Negotiating MOQ with Technical Realities
MOQ terms directly impact total cost of ownership. Avoid these 2026 pitfalls:
- Scanner-Specific Tooling Fees: Custom calibration jigs for CEREC compatibility require $8,500-$12,000 NRE (Non-Recurring Engineering). Negotiate amortization over 3+ orders.
- Software Licensing: Verify if AI-driven margin detection software requires per-unit license fees (typical: $150/scanner). Carejoy includes 3-year licenses in base price.
- 2026 MOQ Strategy:
| Order Volume | Standard Terms | Negotiation Leverage Point |
|---|---|---|
| 1-5 Units | High NRE fees ($12k+), no software inclusion | Request demo unit credit against first production order |
| 6-20 Units | NRE reduced to $5k, 1-year software license | Negotiate extended warranty (Carejoy offers 24 months at 15+ units) |
| 20+ Units | NRE waived, 3-year software license, priority production slot | Lock in 2026 pricing with annual volume commitment (Carejoy provides price stability clauses) |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics
Dental scanners require climate-controlled shipping. Choose terms based on risk tolerance:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (saves 4-7%) | Buyer bears freight, insurance, customs clearance risk. Scanner calibration void if temp exceeds 35°C during transit. | Only for experienced importers with dental logistics partners |
| DDP (Delivered Duty Paid) | Higher unit cost (adds 8-12%) | Supplier manages all risks. Carejoy uses IoT-tracked containers with real-time temp/humidity monitoring (2026 standard). | Strongly recommended – Includes calibration revalidation if thresholds breached |
Strategic Partnership Opportunity: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
As a vertically integrated factory in Baoshan District, Shanghai, Carejoy controls critical processes from optical sensor calibration to CE-compliant software validation. Their 2026 CEREC-compatible scanners feature:
- True 20-micron accuracy (ISO/IEC 17025 certified lab)
- Integrated AI for prep margin detection (FDA-cleared algorithm)
- Direct CEREC Connect compatibility without middleware
For Verified Sourcing:
Contact their International Division:
📧 [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes certificate verifications, calibration protocols, and DDP shipping SLAs
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified case study based on documented compliance records, not an endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: CEREC Scanners – Purchasing Guide 2026
As digital dentistry continues to evolve, CEREC scanners remain a cornerstone of efficient, in-house restorative workflows. This FAQ addresses critical technical and service considerations for clinics and distributors evaluating CEREC scanner procurement in 2026.
| Question | Answer |
|---|---|
| 1. What voltage and power requirements should I verify before purchasing a CEREC scanner in 2026? | Modern CEREC scanners (e.g., Dentsply Sirona CEREC Omnicam AC, Primescan) typically operate on universal voltage input (100–240 VAC, 50/60 Hz), making them compatible with global electrical standards. However, clinics must confirm the specific model’s power adapter rating and ensure stable power supply via a surge-protected outlet. For regions with fluctuating voltage (e.g., parts of Asia, Africa), integration with a line-interactive UPS is recommended to prevent sensor calibration drift and hardware damage. Always verify regional compliance (CE, FDA, TGA, etc.) during procurement. |
| 2. Are spare parts for CEREC scanners readily available, and what components commonly require replacement? | Yes, authorized distributors and Dentsply Sirona service centers maintain inventories of critical spare parts. High-wear components include intraoral tips (disposable or autoclavable), scan bodies, LED light modules, and handpiece cables. As of 2026, OEM part availability is guaranteed for all active models for at least 7 years post-manufacture. Distributors should confirm local stock levels and lead times. Third-party consumables are available but may void warranty if they impact scanner calibration or performance. |
| 3. What does the CEREC scanner installation process involve, and is on-site technician support required? | Installation includes hardware setup (scanner, cart/tablet), software integration with the clinic’s CAD/CAM suite (e.g., CEREC Software 6.0+), DICOM compatibility checks, and network configuration. While basic setup can be user-guided, Dentsply Sirona mandates certified technician installation for warranty validation. The technician performs calibration verification, workflow testing, and staff training (2–4 hours). Remote pre-installation assessments are standard in 2026 to ensure IT infrastructure readiness (minimum 8 GB RAM, SSD, Windows 11 Pro). |
| 4. What warranty coverage is provided with a new CEREC scanner in 2026, and are extended service plans available? | New CEREC scanners come with a standard 2-year comprehensive warranty covering parts, labor, and calibration services. The warranty requires annual preventive maintenance (PM) by an authorized engineer to remain valid. Extended Service Agreements (ESAs) are available for up to 5 additional years, including priority dispatch, predictive diagnostics via cloud monitoring, and coverage for accidental damage. In 2026, over 78% of clinics opt for 3-year ESAs to ensure minimal downtime and predictable budgeting. |
| 5. How are firmware updates and technical support handled post-purchase? | Firmware and software updates are delivered securely through Dentsply Sirona Connect, with automatic notifications and remote installation options. Critical updates (e.g., AI-driven margin detection enhancements) are free during the warranty and ESA periods. 24/7 technical support is available via phone, web portal, and remote desktop assistance. Distributors receive dedicated support SLAs, including spare part loaner programs and on-demand clinical application training for end-users. |
Need a Quote for Cerec Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


