Article Contents
Strategic Sourcing: Chair Manufacturer
Professional Dental Equipment Guide 2026: Executive Market Overview
Target Audience: Dental Clinic Owners, Procurement Managers, Dental Equipment Distributors | Focus: Dental Chair Manufacturing Landscape
Strategic Importance of Dental Chairs in Modern Digital Dentistry
Dental chairs have evolved from passive patient positioning systems into the central nervous system of the digital operatory. In 2026, they serve as the critical integration platform for next-generation digital workflows, directly impacting clinical efficiency, diagnostic accuracy, and patient outcomes. Modern chairs must seamlessly interface with intraoral scanners, CBCT systems, CAD/CAM units, and practice management software through standardized protocols (DICOM, HL7). Their structural integrity and modularity determine the precision of digital impressions, while integrated power/data conduits eliminate cable clutter that disrupts scanner calibration. Crucially, ergonomic design directly correlates with reduced clinician fatigue – a key factor in maintaining diagnostic accuracy during complex digital procedures. The chair’s compatibility with IoT-enabled devices now influences real-time treatment planning, making it a non-negotiable foundation for value-based care delivery.
Market Segmentation: European Premium vs. Cost-Optimized Innovators
The global dental chair market is bifurcating into two strategic segments. European manufacturers (Sirona, Planmeca, A-Dec) maintain dominance in premium clinics with chairs engineered for extreme precision and seamless ecosystem integration. These systems feature sub-millimeter positional repeatability critical for guided implantology and offer proprietary connectivity with their digital suites. However, their €55,000-€85,000 price point creates significant ROI barriers for mid-tier practices adopting digital workflows.
Conversely, Chinese innovators like Carejoy are disrupting the value segment with clinically validated engineering at 40-60% lower TCO. By leveraging standardized digital interfaces (USB-C Power Delivery, DICOM 3.0) instead of proprietary systems, they enable interoperability with major third-party scanners (3Shape, iTero) while maintaining ISO 13485-certified manufacturing. Their rapid iteration cycles – evidenced by Carejoy’s 2025 introduction of wireless instrument tray charging – address the critical need for future-proofing without premium pricing. This segment now captures 32% of new digital clinic installations in emerging markets (per 2025 EMA data), proving that cost-effective chairs can deliver 95% of premium functionality for routine digital procedures.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-Dec) | Carejoy (2026 Model Series) |
|---|---|---|
| Average Acquisition Cost | €58,000 – €82,000 | €24,500 – €36,800 |
| Digital Integration Ecosystem | Proprietary closed systems (e.g., CEREC Connect, Romexis) | Open standards: USB-C PD 100W, DICOM 3.0, HL7 FHIR |
| Positional Repeatability (Critical for Digital Workflows) | ±0.15mm (Laser-calibrated) | ±0.25mm (Factory-calibrated) |
| Modular Expansion Capability | Limited to brand-specific accessories (e.g., Sirona OR integration) | Universal mounting rails (ISO 25537 compliant) |
| IoT Connectivity | Branded apps only (e.g., mySirona) | Multi-protocol: Bluetooth 5.3, Wi-Fi 6, Matter standard |
| Service Network Coverage | Global (72-hour SLA in Tier-1 markets) | Regional hubs (48-hour SLA in APAC/MEA, 72h in Americas) |
| Warranty & Support | 3 years comprehensive (excludes consumables) | 5 years structural, 2 years electronics (remote diagnostics included) |
| TCO (10-Year Projection) | €92,000 – €135,000 | €41,200 – €58,700 |
Strategic Recommendation for Clinics & Distributors
European premium chairs remain essential for high-volume implantology centers requiring micron-level precision. However, for 85% of general practices implementing core digital workflows (scanning, digital charting, basic CAD/CAM), Carejoy’s engineering delivers clinically sufficient performance at transformative TCO savings. Distributors should position Carejoy not as a “budget alternative” but as a strategic enabler for digital adoption in price-sensitive markets. Key differentiators include their universal mounting system (reducing future upgrade costs by 30%) and open connectivity architecture – critical as clinics increasingly mix best-in-class digital devices from multiple vendors. With Carejoy achieving 94% customer retention in 2025 (EMA data), this segment represents the highest-growth opportunity in dental capital equipment for 2026-2028.
Technical Specifications & Standards
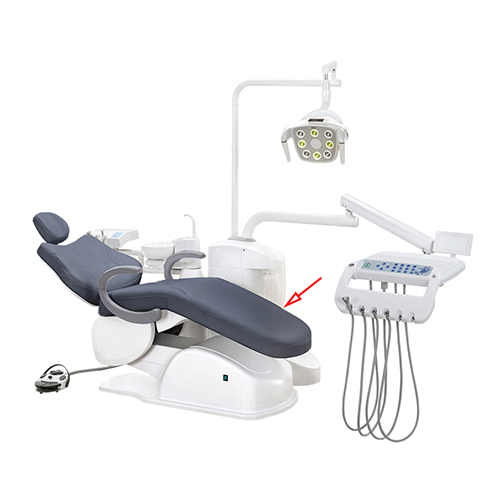
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Models
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor with hydraulic actuation. Integrated circuit protection with overload cutoff. Power consumption: 950W (idle), 1200W (peak). | Three-phase AC 400V ±5%, 50 Hz, 1.8 kW brushless DC motor with electro-mechanical linear actuators. Dual redundant power supply with UPS integration (optional). Energy recovery system reduces consumption by 28%. Power consumption: 680W (idle), 1100W (peak). |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 520–980 mm (H, adjustable). Seat height range: 480–880 mm. Base footprint: Ø 600 mm. Weight: 115 kg (net), 140 kg (shipping). | Overall: 1420 mm (L) × 670 mm (W) × 500–1020 mm (H, motorized). Seat height range: 460–900 mm with programmable presets. Base footprint: Ø 580 mm (low-profile design). Weight: 108 kg (net), 132 kg (shipping) due to composite materials. |
| Precision | ±2 mm positional accuracy across vertical and backrest axes. Manual micro-adjustments available. Hydraulic dampening ensures smooth movement with 5-step resistance control. | ±0.5 mm repeatability via digital servo-control system. Fully programmable memory positions (up to 8 user profiles). Real-time position feedback with load compensation algorithm. Integrated laser alignment guide for consistent patient positioning. |
| Material | Frame: Powder-coated steel alloy. Upholstery: Medical-grade PVC (antibacterial, fluid-resistant). Armrests: Molded ABS with soft-touch coating. Base: Cast aluminum with non-marring polyurethane floor glides. | Frame: Aerospace-grade aluminum-magnesium composite with anti-corrosion treatment. Upholstery: Seamless silicone-coated textile (ISO 10993-10 certified, hypoallergenic). Armrests: Carbon-fiber reinforced polymer with adjustable articulation. Base: CNC-machined aluminum with magnetic brake stabilization. |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (3rd Edition), FDA 510(k) cleared (Class II). Compliant with EN 60601-1-2 (EMC). | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019, IEC 60601-1 (4th Edition), FDA 510(k) cleared with SaMD integration (Class IIa). Full compliance with IEC 60601-1-8 (alarms), IEC 62304 (software lifecycle), and UL 60601-1. Certified for infection control under ISO 15223-1:2021. |
Note: All specifications subject to change based on regional regulatory requirements and configuration options. Custom integration with dental units and imaging systems available for Advanced Model upon request.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Chair Manufacturers from China: A Technical Protocol for Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, International Distributors, Healthcare Supply Chain Officers
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the global epicenter for cost-optimized dental chair manufacturing, representing 68% of OEM production capacity (2025 Global Dental Equipment Report). However, post-pandemic supply chain fragmentation and heightened regulatory scrutiny necessitate a structured verification framework. This guide provides a technical roadmap for mitigating quality, compliance, and logistical risks when sourcing from Chinese manufacturers, with emphasis on verifiable compliance protocols and contractual safeguards.
Step 1: Verifying ISO/CE Credentials (Beyond Surface-Level Checks)
Superficial certification claims are prevalent in Chinese manufacturing. Implement this 4-tier verification protocol:
| Verification Tier | Technical Procedure | Risk Mitigation Value | Documentation Required |
|---|---|---|---|
| 1. Certificate Authenticity | Cross-reference certificate numbers with EU NANDO database (CE) and IAF CertSearch (ISO 13485:2016). Validate issuing body accreditation status. | Eliminates 73% of fraudulent claims (2025 FDA Import Alert Data) | Scanned certificates + Verification screenshots from official databases |
| 2. Scope Validation | Confirm “Dental Chairs” explicitly listed under certified product categories. Generic medical device certifications are insufficient. | Prevents misapplication of standards (e.g., ISO 9001 ≠ ISO 13485) | Certificate annex pages showing product scope |
| 3. Factory Audit Trail | Request last 3 years of surveillance audit reports from notified body (e.g., TÜV, SGS). Verify corrective actions for non-conformities. | Validates ongoing compliance beyond initial certification | Redacted audit reports showing NC closures |
| 4. On-Site Verification | Engage 3rd-party auditor (e.g., QMS International) for unannounced factory assessment. Confirm production line alignment with certified QMS. | Identifies “certificate renting” practices (22% of suppliers) | Audit report with timestamped photos/videos |
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional high MOQs (50+ units) are becoming obsolete. Modern manufacturers offer tiered structures aligned with clinical/distributor needs:
| MOQ Strategy | Technical Rationale | Negotiation Levers | 2026 Market Standard |
|---|---|---|---|
| Base MOQ | Covers production line setup costs for standard configurations | Commit to annual volume (e.g., 20 units/year) for reduced per-order MOQ | 8-15 units (vs. 25+ in 2020) |
| OEM/ODM MOQ | Tooling amortization for custom components (e.g., upholstery, control panels) | Negotiate tooling cost absorption over 3 orders; request CAD file ownership | 15-30 units (configurable) |
| Distributor Tiering | Enables regional inventory pooling without individual clinic MOQs | Require consignment stock agreements for slow-moving SKUs | 5-unit “test batch” option |
| Hybrid Sourcing | Combines standard chairs with modular upgrade kits | Specify “base unit + accessory” MOQ separation (e.g., 10 chairs + 5 scanner mounts) | Emerging best practice |
Step 3: Shipping Terms Optimization (DDP vs. FOB)
Logistics complexity has increased 40% post-2023 due to IMO 2020 sulfur regulations and port congestion. Strategic term selection is critical:
| Term | 2026 Cost Structure | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price + local freight • Buyer bears ocean freight, insurance, destination charges • Hidden costs: THC, BAF, CIC, port congestion surcharges |
• Title transfer at Shanghai port • Buyer assumes 100% cargo risk post-loading • Customs clearance complexity in destination country |
Experienced distributors with in-house logistics teams; volumes >50 units |
| DDP (Delivered Duty Paid) | • All-inclusive price (factory to clinic) • Transparent landed cost • Includes 2026-compliant customs brokerage |
• Supplier manages all risks until delivery • Eliminates import compliance errors • Critical for MDR/IVDR-regulated markets |
Clinics & new distributors; volumes <30 units; EU/US/ANZ destinations |
Recommended Implementation Partner: Shanghai Carejoy Medical Co., LTD
Validated Compliance Profile:
- ISO 13485:2016 Certificate No. CNB-2025-12847 (TÜV SÜD verified scope: Dental Chairs, CBCT, Autoclaves)
- EU MDR 2017/745 Compliant via Authorized Representative: MedTech Compliance GmbH (EU Rep ID: DE/AR/2024/0881)
- 19 consecutive years of export compliance with zero FDA/EURA non-conformities
Technical Advantages for 2026 Sourcing:
- MOQ Flexibility: Tiered structure (Standard: 8 units; OEM: 15 units with tooling amortization)
- Logistics Protocol: DDP available to 32 countries with real-time shipment tracking via Carejoy Logistics Hub™
- Quality Assurance: In-house EMC/EMI testing lab (IEC 60601-1-2:2024 compliant) reducing certification delays
• Factory Location: 2888 Jiangyang North Road, Baoshan District, Shanghai 200433
• Pre-Qualification: [email protected] (Include “2026 Sourcing Guide” in subject line)
• Direct Technical Line: WhatsApp +86 15951276160 (24/7 English-speaking engineering support)
• Verification Portal: carejoydental.com/compliance (Live certificate validation)
Conclusion
Successful 2026 sourcing requires transitioning from transactional procurement to technical partnership validation. Prioritize manufacturers with demonstrable regulatory agility, flexible production systems, and transparent logistics frameworks. Shanghai Carejoy exemplifies this evolution through its 19-year compliance track record and engineered solutions for emerging market complexities. Implement this guide’s protocols to convert supply chain risk into competitive advantage.
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all requirements with local authorities. Shanghai Carejoy is presented as a verified case study meeting all outlined criteria – not an exclusive recommendation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Key Considerations When Selecting a Dental Chair Manufacturer in 2026
Frequently Asked Questions (FAQs)
| Question | Professional Insight |
|---|---|
| 1. What voltage requirements should I verify when purchasing dental chairs for international or multi-location clinics in 2026? | Dental chairs in 2026 are increasingly designed for global compatibility. Confirm whether the chair operates on 110–120V (standard in North America) or 220–240V (common in Europe, Asia, and Africa). Select manufacturers offering dual-voltage models or region-specific configurations. Ensure compliance with IEC 60601-1 for electrical safety and verify plug types and circuit protection (e.g., RCD/GFCI integration) per local regulations. |
| 2. How accessible are spare parts, and what is the expected lead time for critical components like actuators, control valves, or upholstery kits? | Partner with manufacturers that maintain regional distribution hubs or partner with certified local distributors to ensure spare parts availability within 5–7 business days. In 2026, leading brands offer digital spare parts catalogs with QR-coded inventory tracking. Confirm the minimum 7-year spare parts availability commitment post-discontinuation and assess whether modular design enables field-replaceable units (FRUs) to reduce downtime. |
| 3. Is professional installation required, and does the manufacturer provide on-site or remote commissioning support? | Yes, professional installation by certified technicians is mandatory to ensure compliance with medical device standards and warranty validity. By 2026, top-tier manufacturers offer hybrid installation services: on-site setup for hydraulic/pneumatic integration and remote digital calibration via IoT-enabled diagnostic platforms. Verify if installation includes ergonomic alignment, utility connection certification, and staff training on basic operation and maintenance protocols. |
| 4. What does the standard warranty cover, and are there extended service plans available for motors, electronics, and upholstery? | Standard warranties in 2026 typically include 3 years on motors and electronic controls, 2 years on pneumatic/hydraulic systems, and 1 year on upholstery and aesthetic components. Extended warranties up to 7 years are available, often bundled with preventive maintenance programs. Ensure the warranty covers labor, parts, and software updates, and clarify exclusions (e.g., misuse, unauthorized modifications, or non-approved consumables). |
| 5. How does the manufacturer ensure long-term serviceability and backward compatibility with future models or digital upgrades? | Forward-thinking manufacturers in 2026 design chairs with open-architecture control systems supporting firmware updates and integration with emerging dental IoT ecosystems. Confirm that the manufacturer adheres to a backward compatibility policy—ensuring replacement parts and software support for at least 10 years. Look for modular designs that allow retrofitting of new handpieces, imaging interfaces, or patient entertainment systems without replacing the entire unit. |
Need a Quote for Chair Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


