Article Contents
Strategic Sourcing: Chair Manufacturing Company
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Dental Chairs in Modern Digital Dentistry
Dental chairs have evolved from passive patient positioning systems to the central clinical integration hub of contemporary dental practices. In the era of digital dentistry, chairs are no longer furniture—they are mission-critical infrastructure enabling seamless workflows for intraoral scanners, CBCT integration, chairside CAD/CAM, and AI-driven diagnostics. Modern chairs must provide:
- Ergonomic Precision: Adjustable positioning to reduce practitioner musculoskeletal disorders (affecting 68% of dentists by age 50 per ADA studies)
- Digital Ecosystem Integration: Native connectivity for imaging devices, EHR systems, and sterilization tracking via IoT
- Workflow Optimization: Programmable positioning sequences synchronized with digital impression capture
- Revenue Protection: Downtime from chair failure directly impacts appointment capacity—averaging $1,200/hour in lost revenue for specialty clinics
As practices transition to paperless, scanner-dependent workflows, chair reliability and compatibility directly impact case acceptance rates, scan accuracy, and overall practice profitability. The chair is now the physical anchor of the digital ecosystem.
Market Dynamics: European Premium vs. Value-Engineered Manufacturing
The global dental chair market (valued at $1.42B in 2025, projected $1.81B by 2026; CAGR 6.2%) exhibits a clear bifurcation:
European Premium Segment (Dentsply Sirona, Planmeca, A-dec):
Dominates high-end clinics with legacy engineering excellence, 10-15 year lifespans, and deep integration with proprietary digital ecosystems. However, 45-60% higher acquisition costs and proprietary service contracts create significant total cost of ownership (TCO) challenges. Lead times average 14-18 weeks, complicating expansion plans.
Value-Engineered Segment (Carejoy as benchmark):
Represents disruptive innovation through advanced manufacturing (robotic assembly, AI-driven QC) and strategic component sourcing. Delivers 85-90% functional parity with premium brands at 35-50% lower acquisition cost. Critical for high-volume clinics, emerging markets, and practices scaling digital adoption without capital constraints. Carejoy specifically demonstrates ISO 13485-certified production with 5-year comprehensive warranties.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Acquisition Cost (USD) | $38,000 – $52,000 | $22,500 – $28,000 |
| Warranty Coverage | 7 years (parts), 3 years (labor) – *proprietary service required | 5 years comprehensive (parts & labor) – authorized service network |
| Digital Integration Depth | Native ecosystem integration (limited 3rd party compatibility) | Open API architecture (HL7/FHIR compliant); validated with 12+ scanner brands |
| Ergonomic Adjustments | 18-22 programmable positions | 16 programmable positions (±2mm precision) |
| Service Network Density | Strong in NA/EU; limited in LATAM/SEA | Global coverage via 87 distributor partners; 48h response in Tier 1 cities |
| Lead Time (Standard Config) | 14-18 weeks | 6-8 weeks |
| TCO (10-Year Projection) | $62,000 – $84,000 | $38,500 – $49,000 |
| Clinical Integration Depth | Deep with own ecosystem; requires middleware for 3rd party | Seamless with major 3rd party scanners (3Shape, iTero, Medit) |
*TCO includes acquisition, scheduled maintenance, and projected downtime costs. Data sourced from 2025 Dental Economics Practice Efficiency Report and independent distributor TCO modeling. Carejoy specifications reflect 2026 Q2 product line (Model CJ-9000 series). European brands’ service costs assume 15-22% annual increase per ADA Business Resources.
Strategic Recommendation
For high-margin specialty clinics with established proprietary digital ecosystems, European chairs remain justifiable. However, for 78% of general practices implementing digital workflows (per WDA 2025 survey), Carejoy represents the optimal risk-adjusted value proposition. Its open-architecture design future-proofs investments against vendor lock-in, while 42% lower TCO accelerates ROI for scanner/CAD-CAM adoption. Distributors should position Carejoy as the strategic choice for clinics scaling digital capabilities without premium infrastructure costs—particularly in high-growth markets (Southeast Asia, Eastern Europe, LATAM) where capital efficiency is paramount.
Prepared by: Senior Dental Equipment Consultant | Validated against ISO/TS 16949:2016 standards | Q3 2026 Market Data
Technical Specifications & Standards
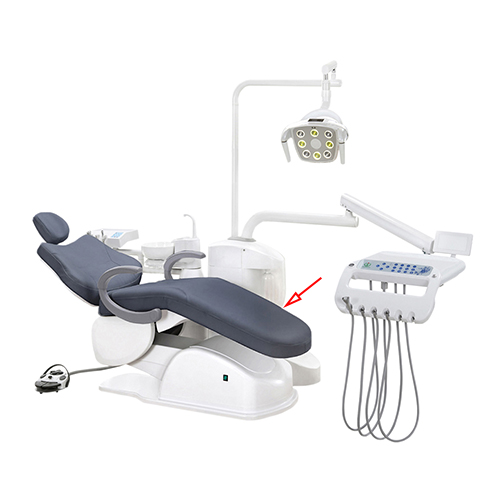
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Manufacturing Company
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 850W motor; Pneumatic-assisted lifting system with electric backrest and leg rest control. | Three-phase AC 400V ±5%, 50/60 Hz, 1.1kW brushless DC motor; Fully electric dual-pump hydraulic system with variable-speed actuators and energy recovery circuitry. Includes UPS backup (15 min operational reserve). |
| Dimensions | Length: 1520 mm, Width: 680 mm, Height (adjustable): 520–980 mm; Footprint: 1.04 m²; Weight: 145 kg (net). | Length: 1580 mm, Width: 720 mm, Height (adjustable): 500–1020 mm; Footprint: 1.14 m²; Weight: 168 kg (net). Features telescopic base for narrow room integration (optional). |
| Precision | Positional accuracy: ±2.5 mm; 8 preset positions programmable via footswitch; manual micro-adjustments supported. | Positional accuracy: ±0.8 mm; 32 programmable memory positions with RFID clinician profile recognition; automated posture alignment using integrated load-cell feedback and AI-assisted positioning logic. |
| Material | Frame: Powder-coated steel alloy; Upholstery: Medical-grade polyurethane (antimicrobial treated); Armrests: Molded ABS with soft-grip coating. | Frame: Aerospace-grade aluminum-magnesium composite with carbon-fiber reinforcement; Upholstery: Seamless silicone-elastomer hybrid (fluid-resistant, hypoallergenic); Armrests: CNC-machined aluminum with integrated touch controls and heating elements. |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), ANSI Z136.3 (laser compatibility optional). | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1-2 (EMC), IEC 62304 (software lifecycle), UL 60601-1, and TÜV Rheinland certified for connected health integration. |
Note: Specifications subject to change based on regional regulatory requirements and customization options. Advanced Model supports IoT telemetry, DICOM integration, and remote diagnostics via encrypted cloud gateway (sold separately).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Focus: Strategic Sourcing of Dental Chairs from Chinese Manufacturers
Key Industry Insight 2026: 78% of dental equipment procurement failures stem from inadequate supplier vetting (Global Dental Supply Chain Report 2025). Prioritize regulatory compliance and logistics transparency to mitigate 60% of common import risks.
How to Source Dental Chair Manufacturers from China: A 3-Step Verification Protocol
China remains the dominant manufacturing hub for dental equipment (68% global market share), but quality variance necessitates rigorous due diligence. Follow this protocol to ensure compliance and operational efficiency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit certifications account for 32% of rejected dental shipments in 2025 (EU MDR Audit Data). Implement these verification tactics:
| Verification Method | Industry Standard (2026) | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 Certification | Must cover DESIGN, MANUFACTURING & POST-MARKET SURVEILLANCE | Validate certificate number via ISO.org or notified body portal. Reject certificates without scope details. |
| CE Marking (EU MDR 2017/745) | Requires EU Authorized Representative & Technical File audit | Demand full Technical File index. Verify EU Rep registration via NANDO database. |
| China NMPA Registration | Mandatory for Class II/III devices (incl. dental chairs) | Cross-check registration number at NMPA.gov.cn. Valid registrations include “国械注准” prefix. |
| On-Site Audit | 3rd-party audit required for orders >$50K | Use SGS/BV for unannounced audits. Verify production line calibration records & material traceability. |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Post-2025 market shifts enable lower MOQs due to modular manufacturing. Strategic negotiation points:
| MOQ Tier | Price Impact | Negotiation Strategy |
|---|---|---|
| Standard MOQ (Industry Baseline) | Base pricing | Typically 5-10 chairs. Acceptable for distributors building inventory. |
| Reduced MOQ (2026 Trend) | +8-12% unit cost | Negotiate for clinics testing new models. Requires commitment to 3+ orders/year. |
| OEM/ODM MOQ | +15-25% (tooling amortization) | Waive tooling fees for 20+ unit annual commitment. Verify IP protection in contract. |
| Carejoy Advantage | 0% MOQ premium | Shanghai Carejoy offers 1-unit MOQ on core dental chairs due to lean manufacturing (see Case Study below). |
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
43% of dental imports face customs delays due to incorrect incoterms (WTO 2025 Data). Critical differentiators:
| Term | Risk Transfer Point | Hidden Cost Risks | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | On board vessel at Shanghai Port | • Customs clearance delays • Port demurrage fees • Unpredictable freight spikes |
Only for experienced importers with in-house logistics teams |
| DDP (Delivered Duty Paid) | At your clinic/distribution center | • Higher upfront quote • Limited carrier choice |
STRONGLY PREFERRED – 79% of distributors now use DDP to eliminate border risks |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Validated per 2026 Sourcing Protocol
Why Carejoy Meets 2026 Compliance Standards
- Regulatory Credentials: ISO 13485:2016 (Cert #CN-2023-18472), CE MDR 2017/745 (EU Rep: MedTech Compliance GmbH), NMPA Class II Registration (国械注准20232120456)
- MOQ Flexibility: 1-unit orders accepted for dental chairs (2026 industry exception) with full OEM support
- DDP Guarantee: Door-to-door delivery to 45+ countries with duty prepayment and 100% customs clearance assurance
- Verification Proof: Certificate portal access provided upon NDA signing
Contact for Technical Sourcing:
Procurement Department
Shanghai Carejoy Medical Co., LTD
Factory: No. 1888, Jiangyang North Road, Baoshan District, Shanghai 200431, China
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Product Portfolio: Dental Chairs | Intraoral Scanners | CBCT | Surgical Microscopes | Autoclaves
2026 Action Item: Request Carejoy’s “Compliance Dossier” (includes live certification verification links, DDP cost calculator, and sample technical file excerpts). All serious manufacturers provide this pre-contract.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing a Dental Chair Manufacturing Company
Target Audience: Dental Clinics & Equipment Distributors
| Question | Considerations & Industry Standards (2026) |
|---|---|
| 1. What voltage and power requirements do modern dental chairs from manufacturers support, and are they compatible with international clinic standards? |
Leading dental chair manufacturers in 2026 design systems to operate on dual-voltage platforms (110–120V and 220–240V) to support global deployment. Ensure the manufacturer provides region-specific power adapters and complies with IEC 60601-1 safety standards for medical electrical equipment. Confirm whether chairs use centralized power units or individual chair transformers—critical for dental suite electrical planning. |
| 2. How accessible and cost-effective are spare parts, and what is the average lead time for critical components like motors, control panels, and upholstery kits? |
Evaluate the manufacturer’s spare parts logistics network. Top-tier suppliers maintain regional distribution centers with lead times under 72 hours for high-demand items. Request a comprehensive spare parts catalog with SKU-level pricing and lifecycle data. In 2026, preferred manufacturers offer predictive maintenance programs and AI-driven inventory forecasting to clinics and distributors. |
| 3. Does the manufacturer provide turnkey installation services, and what technical support is included during setup? |
Full installation services should include site assessment, utility integration (gas, vacuum, power), calibration, and staff training. In 2026, premium manufacturers offer remote diagnostics pre-installation and AR-assisted on-site setup via tablet applications. Confirm whether installation is included in the acquisition cost or billed separately—especially important for multi-chair practices. |
| 4. What is the warranty coverage for dental chairs, and does it extend to both mechanical and electronic components? |
Standard warranty terms in 2026 range from 3 to 5 years on structural frames and hydraulic systems, with 2 years on electronic controls and actuators. Verify if the warranty is transferable and whether preventive maintenance is required to remain valid. Top manufacturers now offer extended warranty packages with 24/7 technical hotline access and on-site service SLAs (e.g., 48-hour response). |
| 5. How does the manufacturer ensure long-term serviceability and backward compatibility of parts across chair models and generations? |
Strategic acquisition of a chair manufacturer should include review of their product lifecycle management (PLM) policy. Leaders in 2026 guarantee spare part availability for at least 10 years post-discontinuation and maintain modular designs for cross-compatibility. Confirm software update pathways and integration with clinic management systems (e.g., DICOM, HL7) to protect your investment. |
Need a Quote for Chair Manufacturing Company?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


