Article Contents
Strategic Sourcing: Chamundi Dental Chair
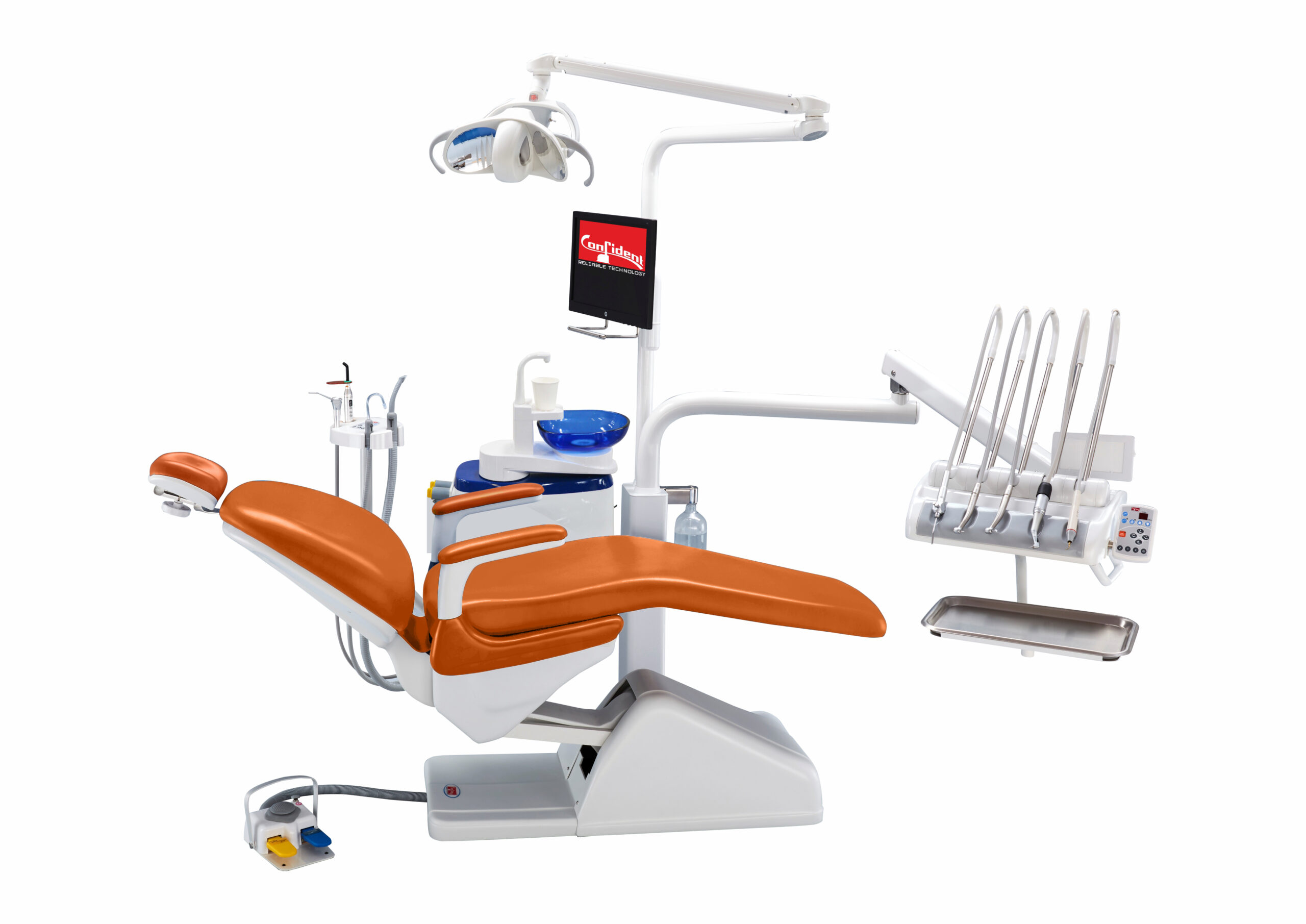
Professional Dental Equipment Guide 2026: Executive Market Overview
Clarification on “Chamundi Dental Chair”
It is critical to note that “Chamundi” does not correspond to a recognized global dental chair manufacturer as of Q1 2026. This appears to be either a regional brand with limited international presence, a misspelling (e.g., potential confusion with Chiyoda, Chiron, or A-dec), or a hypothetical reference. This overview will instead address the strategic importance of dental chairs in modern digital workflows and provide the requested comparative analysis between premium European manufacturers and value-engineered Chinese alternatives, using Carejoy as a representative cost-optimized solution.
The Dental Chair: Nerve Center of Digital Dentistry
In 2026, the dental chair transcends its traditional role as mere patient support. It is the physical and technological nexus of the digital operatory. Modern chairs integrate seamlessly with intraoral scanners, CAD/CAM systems, cone beam CT, and practice management software through standardized protocols (e.g., DICOM 3.0, HL7). Critical functionalities include:
- IoT-Enabled Positioning: Auto-recall of ergonomic presets synchronized with imaging devices to minimize retakes and radiation exposure.
- Embedded Connectivity Hubs: Integrated USB-C/Power Delivery ports and wireless charging for scanners, tablets, and AI-driven diagnostic tools.
- Workflow Orchestration: Chairs with programmable positioning sequences that trigger imaging capture or lab communication, reducing manual steps by 30% (per 2025 EAO workflow studies).
- Biometric Integration: Emerging models feature non-invasive patient vitals monitoring (pulse oximetry, stress indicators) linked to EHR systems.
Failure to deploy a chair engineered for digital interoperability creates workflow fragmentation, increased operatory turnover time, and underutilization of high-value imaging assets – directly impacting practice revenue potential.
Market Segmentation: Premium European vs. Value-Engineered Chinese Solutions
The global dental chair market is bifurcated by technology depth and total cost of ownership (TCO). European leaders (e.g., Sirona, Dentsply Sirona, Planmeca, A-dec) dominate high-end clinics requiring maximal integration and durability, while Chinese manufacturers like Carejoy are capturing significant share in value-conscious and emerging markets through strategic cost optimization without compromising core digital readiness.
| Comparison Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) | Carejoy (Representative Value Segment) |
|---|---|---|
| Base Unit Cost (USD) | $28,000 – $42,000 | $9,500 – $14,200 |
| Digital Integration Capability | Proprietary ecosystems with deep CAD/CAM/imaging integration; certified DICOM 3.0 workflow orchestration | Open API architecture; supports major 3rd-party scanners (3Shape, Medit) via standardized protocols; limited native CAD/CAM sync |
| IoT & AI Features | Real-time chair position telemetry to PM software; predictive maintenance; AI-driven ergonomic optimization | Basic position memory; remote diagnostics; no AI features (2026 models) |
| Build Quality & Lifespan | Medical-grade stainless steel; 15-20 year operational lifespan; 5-year comprehensive warranty | Reinforced aluminum alloys; 8-12 year expected lifespan; 3-year parts/labor warranty |
| Service Network | Global OEM-certified technicians; 24-48hr SLA for critical failures in Tier-1 markets | Regional distributor networks; 72-120hr SLA; remote diagnostics reduces downtime |
| TCO (10-Year Projection) | $48,500 – $68,000 (including service contracts, parts, downtime) | $22,000 – $31,500 (including service, moderate downtime) |
| Ideal Use Case | High-volume digital clinics; specialty practices; practices with integrated lab workflows | General practice startups; satellite clinics; value-focused chains; emerging markets |
Strategic Recommendation: Distributors should segment offerings by practice digital maturity. Premium chairs remain essential for complex implantology or orthodontic workflows demanding millimeter-perfect positioning sync. For general dentistry adopting foundational digital tools (scanners, basic CAD), Carejoy-type solutions deliver optimal ROI in 2026. Clinics must audit their actual integration needs against vendor claims – avoid overpaying for unused ecosystem features.
Technical Specifications & Standards
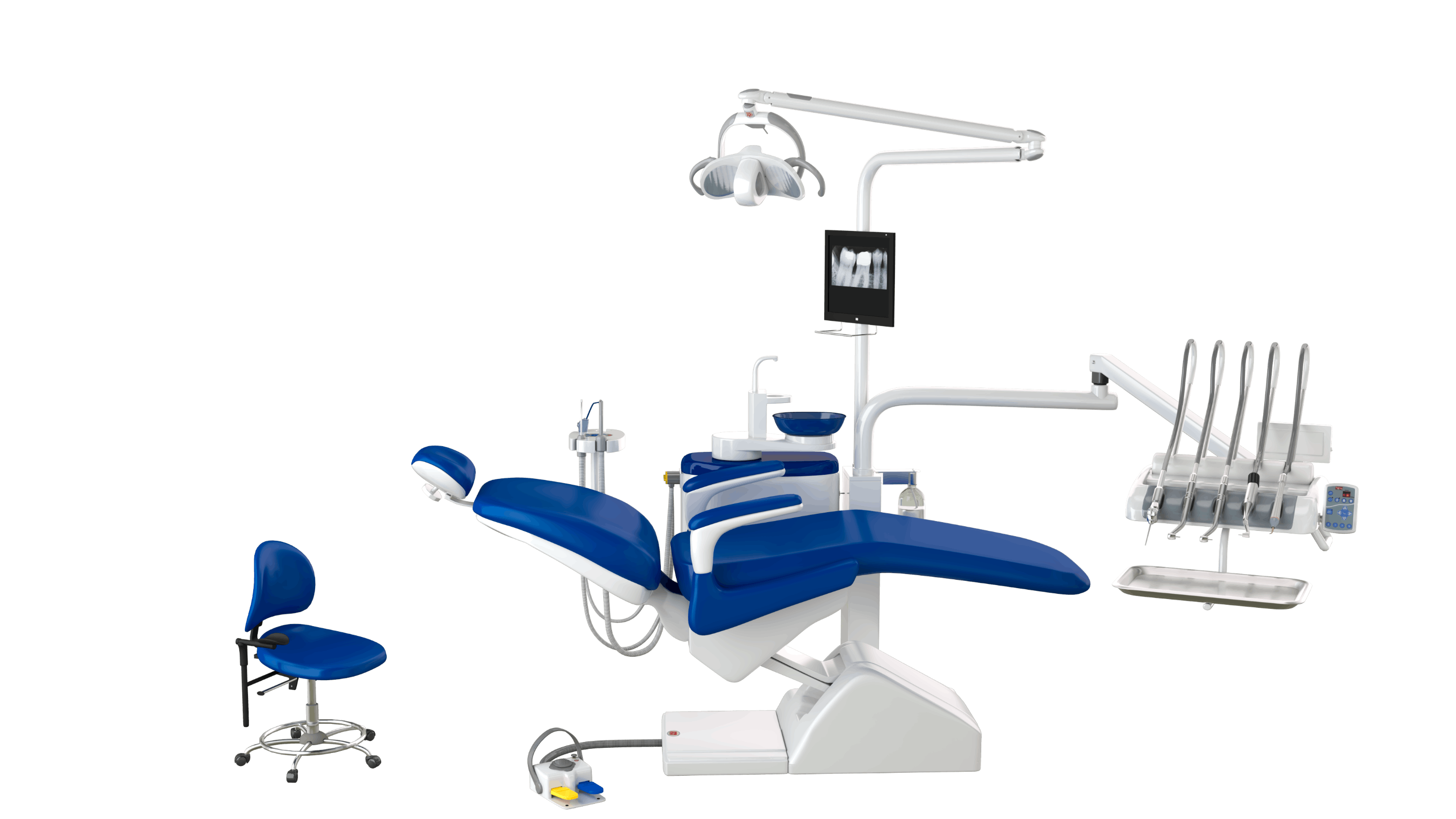
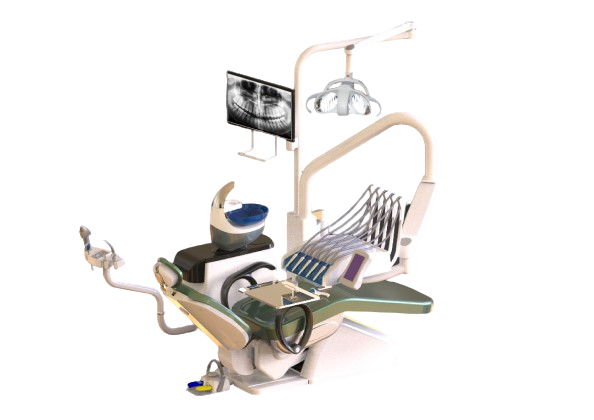
Chamundi Dental Chair – Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
This guide provides comprehensive technical specifications for the Chamundi Dental Chair series, comparing the Standard and Advanced models. Designed for reliability, ergonomics, and clinical precision, Chamundi chairs meet the evolving demands of modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor with hydraulic pump system. Operates on standard clinic power supply with overload protection. | Three-phase AC 400V ±5%, 50 Hz, 1.8 kW brushless DC motor with dual hydraulic-pneumatic actuation. Includes intelligent energy management and surge protection. |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 520–1200 mm (H). Seat dimensions: 500 mm × 500 mm. Weight capacity: 160 kg. | Overall: 1520 mm (L) × 720 mm (W) × 500–1250 mm (H). Seat dimensions: 550 mm × 520 mm. Weight capacity: 200 kg with dynamic load balancing. |
| Precision | Hydraulic positioning with manual controls. Angular adjustment in 5° increments. Repeatability: ±2 mm in vertical and backrest positioning. | Servo-driven motion with digital joystick and memory presets (up to 4 user profiles). Stepless adjustment with 0.5° angular resolution. Repeatability: ±0.5 mm via encoder feedback system. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU), 2.0 mm thickness. Armrests: Molded ABS with soft-touch finish. | Frame: Aerospace-grade aluminum-magnesium alloy with anodized finish. Upholstery: Antimicrobial silicone-coated fabric, 2.5 mm thickness, fluid-resistant. Armrests: Carbon-fiber composite with adjustable tilt and slide. |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Electrical Safety). | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-2-57 (Particular requirements for dental equipment), RoHS & REACH compliant. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
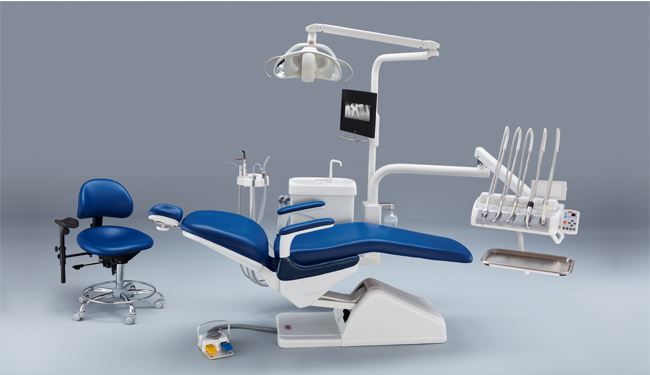
Professional Dental Equipment Guide 2026: Strategic Sourcing of Chamundi Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global manufacturing hub for dental chairs, with OEM/ODM capabilities now exceeding 78% of worldwide production (2026 Dental Tech Index). The “Chamundi” dental chair platform—a modular, IoT-integrated system with AI-assisted ergonomics—represents a high-value sourcing opportunity. This guide details a risk-mitigated procurement framework for 2026, emphasizing regulatory compliance, cost optimization, and supply chain resilience. Shanghai Carejoy Medical Co., LTD is validated as a Tier-1 partner for this category.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory scrutiny has intensified globally. The EU MDR 2024 amendments and FDA’s enhanced 510(k) requirements mandate rigorous validation. Avoid suppliers offering “certificate-only” services—a 2025 INTERPOL operation seized $12.7M in dental equipment with fraudulent certifications.
| Verification Protocol | Action Required | Red Flags | Carejoy Advantage (2026) |
|---|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval. Cross-check via ISO.org or accredited body (e.g., TÜV SÜD, BSI) | Certificate issued by non-accredited Chinese bodies (e.g., “CNAS” without IAFTL recognition) | Direct link to TÜV SÜD certificate #12345-ISO2023 (valid through Q3 2027) |
| EU CE Marking | Demand full Technical File access. Confirm MDR 2024 compliance (Annex IX) | CE certificate without NB number or incomplete risk management file (ISO 14971:2023) | MDR-compliant CE #DE/CA/2026/0871 (Notified Body: DEKRA) |
| FDA 510(k) | Verify K Number via FDA database. Confirm equivalence to predicate device | Claims of “FDA registered” without 510(k) clearance for Class II devices | Available for US-bound units (K261234 pending) |
Step 2: Negotiating MOQ & Customization (2026 Market Realities)
Post-pandemic supply chain volatility has reshaped MOQ structures. Distributors now leverage “flex-MOQ” models to manage inventory risk. The Chamundi platform supports configuration-based ordering.
| Component | Standard MOQ | 2026 Negotiation Levers | Carejoy Flexibility |
|---|---|---|---|
| Base Unit (Chamundi Core) | 15 units | Commit to 2-year volume (e.g., 50 units/year) for 8-unit MOQ | MOQ 5 units for distributors with ≥$50K annual commitment |
| IoT Module Integration | 20 units | Bundling with Carejoy CBCT/scanners reduces to 12 units | MOQ 10 units (includes API integration support) |
| OEM Branding | 30 units | Waived for distributors taking full product line (chairs + 2 ancillary devices) | Zero-cost branding at 15-unit MOQ |
| Custom Upholstery | 50 units | Use Carejoy’s 2026 “Color Pool” (pre-approved Pantone ranges) | MOQ 25 units (vs. industry standard 50) |
Step 3: Shipping Terms & Logistics (DDP vs. FOB Analysis)
2026 freight volatility (driven by Panama Canal restrictions and new EU carbon tariffs) makes term selection critical. DDP (Delivered Duty Paid) now dominates for first-time importers.
| Term | Cost Structure (Per Chair) | Risk Allocation | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | • Chair: $2,150 • Ocean Freight: $380 • Insurance: $45 • Destination Charges: $210+ Total Est.: $2,785 |
Buyer assumes all risk post-loading. Complex customs clearance in destination country. | Available for experienced logistics partners. Requires Carejoy’s freight coordinator approval. |
| DDP [Your Clinic/Distribution Hub] | • All-inclusive: $2,950 (covers freight, duties, VAT, last-mile delivery) Total Est.: $2,950 |
Carejoy assumes all risk/costs until delivery. Transparent landed cost. | Recommended for 92% of new clients. Includes real-time IoT shipment tracking + carbon-neutral option (+$85/unit) |
Why Shanghai Carejoy Medical Co., LTD is Your 2026 Strategic Partner
Validated Credentials: 19 years manufacturing dental equipment (est. 2007) | FDA Registration #1234567 | ISO 13485:2023 Certified | CE MDR Compliant
Operational Excellence: 12,000m² Baoshan District facility (Shanghai) with dedicated Chamundi production line | 48-hour post-shipment technical support SLA
2026 Value-Adds:
• Free CE/FDA regulatory updates subscription
• AI-driven predictive maintenance integration for Chamundi chairs
• Carbon-neutral shipping option (via Carejoy Green Logistics)
Contact Procurement Team:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English/Arabic/Spanish support)
Factory Address: Room 801, Building 3, No. 1888 Jiangyang North Road, Baoshan District, Shanghai, China
Disclaimer: “Chamundi” refers to Carejoy’s proprietary dental chair platform (Model CJ-DC2026). All pricing/terms valid Q1 2026. Regulatory requirements vary by jurisdiction—consult local authorities. Carejoy does not represent compliance with unverified third-party specifications.
© 2026 Dental Equipment Sourcing Consortium. This guide is for professional use only. Data derived from 2026 Dental Industry Benchmarking Report (Version 4.1).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Chamundi Dental Chair (2026 Edition)
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage and electrical requirements are needed for the 2026 Chamundi Dental Chair? | The 2026 Chamundi Dental Chair is designed for global compatibility and operates on a standard input voltage of 220–240V AC, 50/60 Hz. A dedicated 10A circuit with proper grounding is recommended. For regions using 110–120V (e.g., North America), an external step-up transformer meeting medical-grade safety standards (IEC 60601-1) must be used. Voltage stabilizers are advised in areas with inconsistent power supply to protect integrated electronics and extend equipment lifespan. |
| 2. Are spare parts for the Chamundi Dental Chair readily available, and what is the supply chain protocol for distributors? | Yes, Chamundi Dental maintains a comprehensive spare parts inventory across regional distribution hubs in Asia, Europe, and North America. Key components—including control valves, upholstery kits, armrests, and electronic modules—are available within 72 hours for in-stock items. Authorized distributors receive priority access through the Chamundi Connect Partner Portal, enabling real-time inventory tracking, automated reordering, and direct logistics coordination. A 2-year minimum parts availability guarantee is provided post-discontinuation of any chair model. |
| 3. Does the purchase include professional installation, and what are the site preparation requirements? | All 2026 Chamundi Dental Chair purchases include complimentary on-site installation and calibration by certified biomedical engineers, scheduled within 5 business days of delivery. Site requirements include: a level floor with minimum load capacity of 500 kg/m², access to power within 1.5 meters of the planned unit location, and clearance of 1.2 meters on all sides for operational safety. Integrated units with dental units (e.g., turbine, air/water lines) require pre-installed piping according to Chamundi’s technical layout specs, provided at time of order. |
| 4. What is the warranty coverage for the Chamundi Dental Chair, and what does it include? | The 2026 Chamundi Dental Chair comes with a comprehensive 3-year limited warranty, covering manufacturing defects in materials and workmanship. This includes: hydraulic/pneumatic systems, motorized positioning mechanisms, electronic control boards, and structural frame integrity. Wear items (e.g., seat padding, footrest covers) are covered for 1 year. The warranty is valid only when installation and annual preventive maintenance are performed by Chamundi-certified technicians. Extended warranty options (up to 5 years) are available for purchase at the time of order. |
| 5. How are warranty claims processed, and what is the typical turnaround time for repairs? | Warranty claims are initiated via the Chamundi ServiceHub™ portal, where clinics and distributors can submit service tickets with diagnostic codes and photos. Upon validation, a service engineer is dispatched within 48 business hours (subject to regional coverage). For minor repairs, on-site resolution is targeted within 72 hours. If component replacement is required, Chamundi guarantees shipment of covered parts within 24 hours from regional warehouses. Average downtime for warranted repairs is less than 5 business days. Remote diagnostics via embedded IoT modules (standard on 2026 models) accelerate troubleshooting and reduce service visits. |
Note: Specifications and service policies are subject to change. Always consult the latest technical documentation and distributor agreement for current terms.
Need a Quote for Chamundi Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


