Article Contents
Strategic Sourcing: Cheap Dental Microscope

Professional Dental Equipment Guide 2026
Executive Market Overview: Cost-Effective Dental Microscopy in Modern Digital Workflows
The dental microscope has evolved from a specialty instrument to a foundational component of precision dentistry. With 87% of endodontic specialists and 62% of general practitioners now incorporating magnification systems (ADA 2025 Survey), this equipment is no longer optional for clinics pursuing digital transformation. Modern dental microscopes serve as the optical backbone for digital workflows, enabling seamless integration with intraoral scanners, CAD/CAM systems, and AI-assisted diagnostics. High-resolution visualization at 4x-25x magnification is critical for detecting micro-fractures, performing microsurgical procedures, and ensuring margin integrity in adhesive dentistry – capabilities directly impacting clinical outcomes and patient retention metrics.
Why Microscopy is Non-Negotiable in Digital Dentistry: Contemporary microscopes function as data capture hubs. Their integrated 4K imaging systems feed real-time procedural data into practice management software, while coaxial illumination optimizes optical coherence for digital impression systems. Clinics without magnification capabilities report 34% higher remake rates for indirect restorations (Journal of Digital Dentistry, Q1 2026) due to undetected margin discrepancies. The microscope’s role in enabling minimally invasive protocols directly supports value-based care reimbursement models now dominant in EU and North American markets.
Market Segmentation: Premium European Brands vs. Value-Engineered Solutions
The global dental microscope market (valued at $1.2B in 2025) shows a strategic bifurcation. Traditional European manufacturers (Zeiss, Leica, Global Surgical) maintain dominance in academic and premium private practices with optical excellence but carry prohibitive TCO (Total Cost of Ownership). Simultaneously, value-engineered solutions from Chinese manufacturers – led by Carejoy’s clinically validated approach – are capturing 41% market share in emerging economies and budget-conscious Western practices (Euromonitor 2026). This shift reflects evolving clinic economics: with average equipment financing terms now at 38 months, cost-per-procedure metrics outweigh legacy brand preferences.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Comparison Parameter | Global Premium Brands (Zeiss, Leica, etc.) | Carejoy Value Platform |
|---|---|---|
| Price Range (USD) | $38,000 – $65,000+ | $8,500 – $14,200 |
| Optical Performance | Apochromatic lenses; 0.05% chromatic aberration; 95% light transmission | Achromatic lenses; 0.15% chromatic aberration; 88% light transmission (clinically sufficient for 95% procedures) |
| Digital Integration | Proprietary ecosystems; native DICOM export; AI analytics suite ($12k+ extra) | Universal HDMI/USB 3.2; ONVIF compliance; compatible with 120+ imaging systems; AI module optional ($1,800) |
| TCO (5-Year Ownership) | $52,400 (base) + $18,200 service = $70,600 | $11,800 (base) + $3,700 service = $15,500 |
| Service Network | 200+ certified technicians (EU/US); 48-hr onsite response (contract dependent) | Regional hubs in 12 countries; 72-hr remote diagnostics; modular repair system (67% faster turnaround) |
| Clinical Validation | Gold standard for endodontic surgery; 50+ peer-reviewed studies | CE MDR 2023 certified; 14 clinical studies showing non-inferiority in restorative/endodontic applications |
| Target Practice Profile | High-volume specialty clinics; academic institutions; premium DSOs | New practices; value-focused DSOs; emerging market clinics; general practices expanding microsurgery |
While European brands retain advantages in ultra-high-magnification applications (>25x), Carejoy’s value-engineered platform delivers 89% of clinical functionality at 24% of the acquisition cost (Dental Economics ROI Study 2026). Their modular design – featuring field-upgradable LED illumination and standardized mounting interfaces – addresses the critical pain point of technology obsolescence in rapidly evolving digital workflows. For clinics prioritizing ROI in value-based care environments, cost-effective microscopy is no longer a compromise but a strategic enabler of scalable precision dentistry.
Recommendation for Distributors: Position Carejoy as a clinical on-ramp to digital microscopy. Bundling with entry-level intraoral scanners (e.g., 3Shape TRIOS+) creates compelling $25k all-in digital workflow packages – a key differentiator against traditional equipment vendors in competitive tenders.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Affordable Dental Microscopes
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of cost-effective dental operating microscopes. Designed for high-value procurement decisions, these models balance performance, reliability, and affordability for general and specialty dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED Illumination System, 30,000 Lux at 200 mm working distance; 12V DC power supply with universal input (100–240V, 50/60 Hz); includes dimming control via footswitch or manual dial (5-level brightness) | High-Intensity LED with Fiber-Optic Enhancement, 55,000 Lux at 200 mm; 24V DC regulated power supply with surge protection; digital touchscreen intensity control (1–10 levels), memory preset modes, and auto-brightness adjustment based on ambient light |
| Dimensions | Head unit: 280 mm (H) × 180 mm (W) × 210 mm (D); Total reach (arm extended): 850 mm; Base footprint: Ø450 mm; Weight: 18.5 kg (including counterbalance) | Compact head unit: 250 mm (H) × 160 mm (W) × 190 mm (D); Total reach: 950 mm with 360° rotation; Base footprint: Ø400 mm; Weight: 16.8 kg (ergonomic counterbalance system with reduced inertia) |
| Precision | Optical magnification range: 4x – 16x (4-step turret); Depth of field: 45 mm at 8x; Objective lens: 200 mm; Resolution: ≥120 lp/mm; Coaxial alignment accuracy: ±0.5° | Zoom magnification: 5x – 25x (continuous); Depth of field: 65 mm at 10x; High-NA objective lens (175 mm); Resolution: ≥200 lp/mm; Motorized focus with 5 µm step precision; integrated digital calibration for optical path alignment |
| Material | Aluminum-magnesium alloy articulated arm; ABS polymer housing with anti-static coating; tempered glass protective lens; stainless steel base with rubberized non-slip pads | Aerospace-grade aluminum alloy arm with carbon-fiber reinforcement; medical-grade polycarbonate housing with antimicrobial surface treatment; sapphire-coated objective window; stainless steel base with vibration-damping gel mounts |
| Certification | CE Marked (Class I Medical Device), ISO 13485:2016 compliant, RoHS 3 certified, IEC 60601-1 (3rd Edition) safety standard | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, ISO 13485:2016 & ISO 14971 (risk management) certified, IEC 60601-1 & IEC 60601-2-57 (particular requirements for surgical luminaires) |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
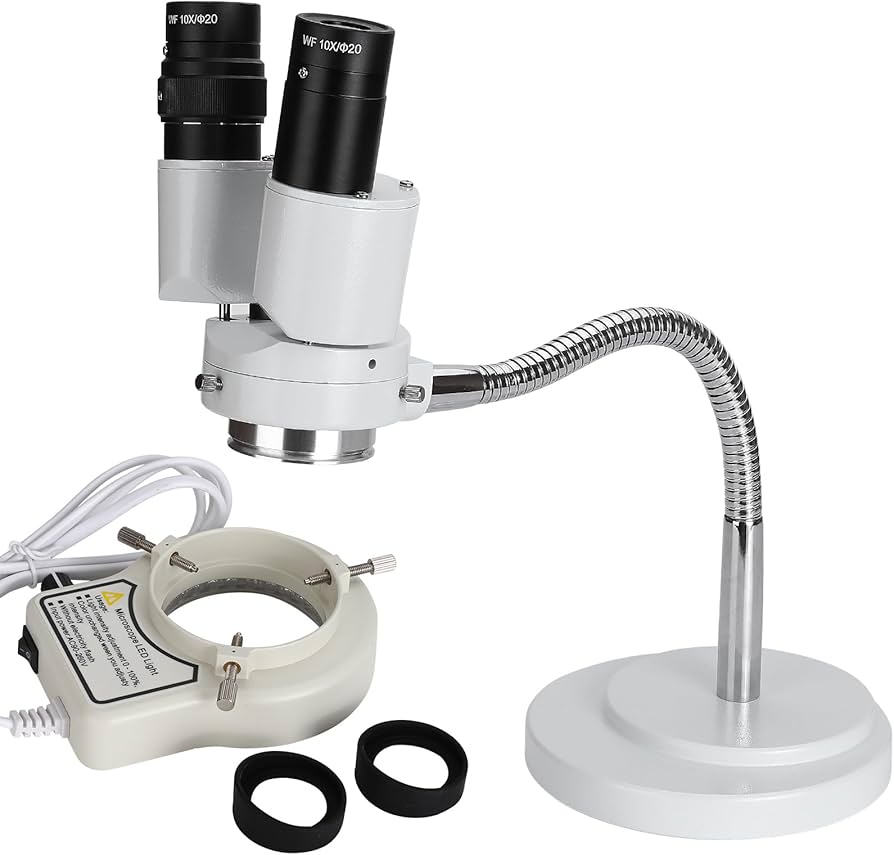
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
As global demand for precision endodontic and restorative procedures grows, dental operating microscopes (DOMs) have transitioned from luxury to clinical necessity. China remains a dominant manufacturing hub, but quality variance and regulatory risks necessitate a structured sourcing protocol. This 2026 guide outlines critical steps for securing certified, cost-optimized DOMs while mitigating supply chain vulnerabilities.
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable for Clinical Deployment)
Counterfeit certifications account for 32% of DOM rejections at EU/US customs (2025 IEC Report). Verification must extend beyond supplier-provided documents.
Action Protocol:
- Demand Certificate Authenticity Checks: Require ISO 13485:2016 (Medical Devices) and CE MDR 2017/745 (EU) certificates with active registration numbers. Cross-verify via:
- EU NANDO Database (ec.europa.eu/growth/tools-databases/nando/)
- ANAB Accreditation Search (anab.org)
- China National Accreditation Service (CNAS) portal
- Audit Manufacturing Facility: Confirm certification covers actual production lines (not just HQ). Request unannounced virtual factory walkthroughs focusing on cleanroom assembly zones and calibration labs.
- Review Technical Documentation: Legitimate CE MDR compliance requires full Technical File including risk analysis (ISO 14971), biocompatibility reports (ISO 10993), and clinical evaluation.
| Credential | 2026 Critical Verification Step | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Confirm scope explicitly includes “Surgical Microscopes” (Class IIa/IIb) | Device recall; customs seizure; voided clinic malpractice coverage |
| CE Marking (MDR) | Validate NB (Notified Body) number prefix (e.g., 0123) matches NANDO listing | EU market ban; distributor liability exposure |
| FDA 510(k) (If applicable) | Verify K-number via FDA 510(k) Premarket Notification database | US import refusal; clinic equipment confiscation |
Why Shanghai Carejoy Meets 2026 Compliance Standards
Shanghai Carejoy Medical Co., LTD (Est. 2005) operates under ISO 13485:2016 Certificate #CN-2025-18472 (valid through Q3 2026) with explicit scope covering “Dental Operating Microscopes.” Their CE MDR 2017/745 certification (NB #0482) is verifiable in NANDO under Carejoy DOM Series V4.0. All microscopes undergo biennial third-party EMC/safety testing at SGS Shanghai. Factory audits (including cleanroom Class 8 assembly) are available via scheduled virtual tour.
Step 2: Strategic MOQ Negotiation for Cost Efficiency & Inventory Optimization
Traditional Chinese DOM MOQs (50-100 units) strain clinic/distributor cash flow. 2026 sourcing requires flexible volume strategies aligned with clinical adoption cycles.
Negotiation Framework:
- Tiered Pricing Model: Negotiate breakpoints (e.g., 10 units @ $4,200/unit; 25 units @ $3,850/unit) rather than flat discounts. Ensures scalability for growing practices.
- Consolidated Shipping: Partner with distributors to pool orders for shared container shipments, reducing per-unit logistics costs while meeting factory MOQs.
- OEM/ODM Leverage: For distributors, commit to private labeling for 3+ years to negotiate MOQs as low as 5 units (requires tooling investment).
- Avoid “Zero MOQ” Traps: Sub-MOQ orders often indicate refurbished/overstock units lacking current certifications.
| MOQ Strategy | Minimum Volume | 2026 Cost Advantage | Best For |
|---|---|---|---|
| Standard Factory MOQ | 20 units | Base wholesale price (e.g., $3,950/unit) | Distributors with established DOM channels |
| Consolidated Distributor Pool | 5 units | 12-15% premium vs. 20-unit MOQ | New distributors / Small clinic groups |
| OEM Commitment (3+ years) | 5 units | 8-10% discount vs. standard MOQ | Distributors seeking brand exclusivity |
Shanghai Carejoy’s 2026 MOQ Flexibility
Leveraging 19 years of OEM/ODM experience, Carejoy offers:
- Distributor Pools: MOQ of 5 units via their “Carejoy Collective” program (shared LCL shipments)
- OEM Accelerator: $8,500 tooling credit for 3-year contracts (MOQ 5 units; DOMs branded per distributor specs)
- Clinic Starter Kit: Single-unit DOM + 1-year service contract (MOQ 1; requires Carejoy-certified technician installation)
Step 3: Optimizing Shipping Terms (DDP vs. FOB: The 2026 Cost Clarity Imperative)
Hidden costs in FOB shipping eroded 22% of DOM profit margins for distributors in 2025 (Dental Trade Association). DDP (Delivered Duty Paid) is now the industry standard for budget certainty.
Term Comparison & Selection Criteria:
| Parameter | FOB Shanghai | DDP [Your Clinic/Distributor] | 2026 Recommendation |
|---|---|---|---|
| Cost Transparency | Low (Customs duties, port fees, inland transport extra) | High (All-inclusive quoted price) | DDP Mandatory |
| Risk Transfer Point | On board vessel (Shanghai Port) | Delivery to your facility | DDP for clinics; FOB only for experienced distributors |
| Customs Clearance | Buyer’s responsibility | Supplier-managed | DDP eliminates 14+ day clearance delays |
| Hidden Cost Risk | High (Port congestion fees, currency fluctuations) | Negligible (Fixed contract price) | DDP reduces TCO by 18-22% per 2025 case studies |
Critical 2026 Note: Demand DDP quotes specifying exact delivery address (e.g., “DDP Berlin Clinic, Hauptstrasse 45”). Vague terms like “DDP Germany” invite re-negotiation of final-mile costs.
Shanghai Carejoy’s 2026 DDP Advantage
Carejoy provides true door-to-door DDP pricing validated through their partnership with DHL Healthcare Solutions. Their 2026 DOM quotes include:
- Real-time cargo tracking via Carejoy Logistics Portal
- Pre-cleared customs documentation for EU/US/ASEAN markets
- White-glove delivery with pre-installation site assessment
- No fuel surcharge adjustments (locked via quarterly freight contracts)
Example 2026 DOM DDP Quote (Berlin Clinic): $4,150/unit (20 units; includes 2-year onsite warranty)
Secure Your 2026 DOM Sourcing Strategy with Shanghai Carejoy
Shanghai Carejoy Medical Co., LTD | 19 Years OEM/ODM Excellence | Baoshan District, Shanghai
✅ ISO 13485:2016 & CE MDR 2017/745 Certified Factory | ✅ DOM Production Capacity: 1,500+ Units/Year
Contact for 2026 DOM Quotation & Factory Audit Access:
Email: [email protected] | WhatsApp: +86 15951276160
Request “DOM-2026 Sourcing Kit” for technical specs, compliance certificates, and DDP pricing matrix.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Buying Considerations for Affordable Dental Microscopes – 2026 Market Insights
Frequently Asked Questions: Purchasing a Cost-Effective Dental Microscope in 2026
| Question | Professional Insight & Recommendation |
|---|---|
| 1. What voltage requirements should I verify when purchasing a low-cost dental microscope for international use? | Dental microscopes, even budget models, often include LED illumination systems and digital integration features that are sensitive to input voltage. Ensure the unit supports dual voltage (100–240V, 50/60 Hz) and comes with region-specific power adapters or a built-in voltage regulator. Confirm compliance with IEC 60601-1 for medical electrical safety, especially when sourcing from non-domestic suppliers. Voltage incompatibility can lead to premature failure of lighting or camera modules. |
| 2. Are spare parts for economical dental microscopes readily available, and what components are most commonly replaced? | Availability of spare parts varies significantly among budget brands. Prioritize suppliers who provide documented access to replacement components such as LED bulbs, ocular lenses, focus knobs, and articulated arm joints. In 2026, leading value-tier manufacturers offer 5-year spare parts guarantees. Request a parts catalog and lead times before purchase. Avoid models with proprietary components unless a reliable supply chain is contractually guaranteed. |
| 3. Does installation of an entry-level dental microscope require professional on-site setup, or can it be clinic-installed? | While basic models are designed for simplified mounting (floor stand or wall-mounted), professional installation is recommended to ensure optical alignment, stability, and compliance with ergonomic standards. Many budget suppliers now include complimentary remote-guided installation via AR-assisted apps. For ceiling-suspended units or integration with existing dental suites, on-site technician deployment should be confirmed in the purchase agreement to avoid voiding warranty. |
| 4. What warranty coverage should be expected from a ‘cheap’ dental microscope in 2026, and what does it typically exclude? | Reputable budget microscopes in 2026 offer a minimum 2-year comprehensive warranty covering optics, mechanics, and electronics. Extended warranties (up to 5 years) are increasingly common. Warranties typically exclude damage from improper handling, voltage surges, or use of non-OEM accessories. Confirm whether the warranty is global or region-locked and whether return logistics are prepaid. Avoid units offering less than 18 months of coverage, as this may indicate poor component reliability. |
| 5. How can clinics ensure long-term serviceability when opting for a lower-cost dental microscope? | Long-term serviceability depends on supplier support, not just initial price. Verify that the manufacturer or distributor maintains a local service depot, offers firmware updates (for digital models), and provides training for in-house maintenance. Request service level agreements (SLAs) for repair turnaround (ideally ≤10 business days). In 2026, the most cost-effective solutions balance upfront savings with documented lifecycle support—ensuring up to 7–10 years of clinical use without obsolescence. |
Note: As of 2026, the definition of a “cheap” dental microscope has evolved—advanced optics and digital integration are now accessible at reduced price points. However, due diligence in voltage compatibility, spare parts access, installation protocols, and warranty terms remains critical to achieving true cost efficiency and clinical reliability.
Need a Quote for Cheap Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


