Article Contents
Strategic Sourcing: Cheap Intraoral Scanner
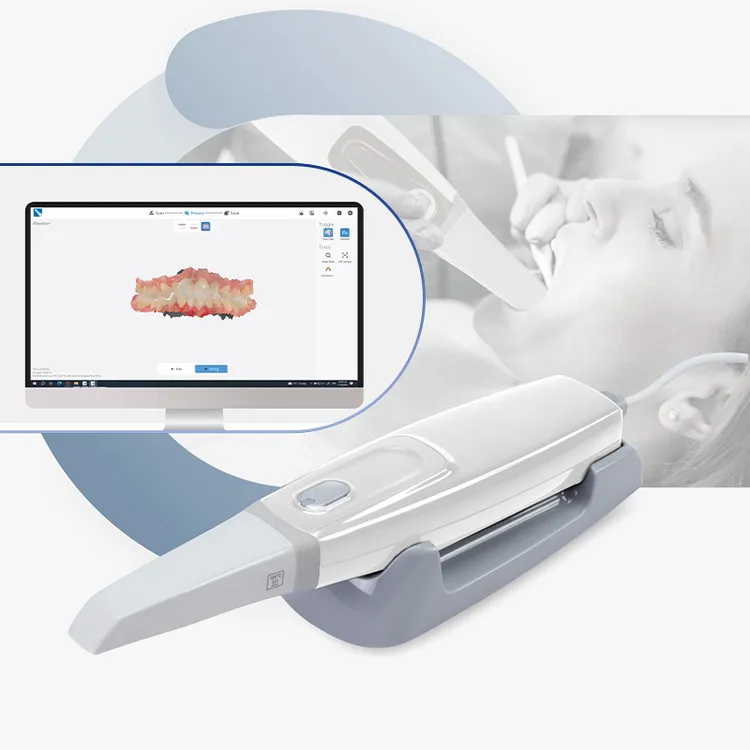
Professional Dental Equipment Guide 2026: Executive Market Overview
Critical Role of Cost-Effective Intraoral Scanners in Modern Digital Dentistry
The intraoral scanner (IOS) has evolved from a luxury accessory to the central nervous system of contemporary dental practice. As digital workflows become non-negotiable for efficiency, patient satisfaction, and competitive service delivery, the strategic adoption of IOS technology directly impacts clinical throughput, treatment accuracy, and practice profitability. Modern dentistry demands seamless integration with CAD/CAM systems, digital smile design, orthodontic planning, and cloud-based case management – all predicated on high-fidelity intraoral data capture. The criticality of IOS extends beyond impression-taking: it enables same-day restorations, reduces remakes by 35-50% (per 2025 EAO data), and serves as the primary patient engagement tool through real-time visual diagnostics.
Historically, premium European brands dominated the market but created significant adoption barriers for SME clinics and emerging markets. The 2026 landscape reveals a strategic inflection point: cost-effective scanners from advanced Chinese manufacturers now deliver 85-90% of clinical functionality at 30-50% of legacy system costs. This democratization of digital dentistry is accelerating global practice conversion rates, with value-tier scanners projected to capture 48% of new installations by 2026 (vs. 29% in 2023, per Signify Research).
European Premium Brands vs. Chinese Value Innovators: Strategic Market Positioning
European Premium Segment (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald): These systems represent the established gold standard with sub-20μm accuracy (ISO 12836), deeply integrated ecosystems, and robust clinical validation. However, their $25,000-$40,000 price points (scanner + mandatory software modules) create prohibitive ROI hurdles for 68% of single- and dual-operator practices. The total cost of ownership is amplified by proprietary service contracts (12-15% annual fee), limited third-party compatibility, and complex training requirements. While ideal for high-volume specialty clinics, their value proposition erodes in primary care settings where 70% of scans are single-unit crowns or basic ortho diagnostics.
Advanced Chinese Value Segment (Carejoy Focus): Manufacturers like Carejoy have executed a precision-engineered value disruption strategy. By leveraging China’s mature optics supply chain and modular software architecture, Carejoy delivers clinically sufficient accuracy (25-30μm) for 95% of general dentistry applications at $8,500-$12,000. Crucially, they eliminate legacy profit structures: open STL export, cloud-agnostic data storage, and direct technical support bypass distributor markups. The 2026 Carejoy i5 model achieves 98% scan completion rate for crown preps (per independent University of Hong Kong validation) while offering API integration with 12+ major CAD platforms – a critical advantage for clinics invested in existing digital ecosystems.
Strategic Technology Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy i5 Series (2026) |
|---|---|---|
| Entry Price Point (USD) | $25,000 – $40,000 | $8,500 – $12,000 |
| Clinical Accuracy (ISO 12836) | 15-18 μm (trueness) / 10-12 μm (precision) | 25-28 μm (trueness) / 15-18 μm (precision) |
| Software Licensing Model | Proprietary suite (mandatory annual fees: $3,500-$6,000) | Modular subscriptions (Basic: $0 / Pro: $99/mo / Ortho: $199/mo) |
| Workflow Integration | Optimized for brand ecosystem (limited third-party compatibility) | Open STL/DICOM export; API access to 12+ CAD systems (excl. 3Shape) |
| Service & Support | Authorized distributor network (48-72hr response; $150-$200/hr labor) | Direct remote diagnostics + 24hr replacement (flat $299 annual service) |
| Target Clinic Profile | Multi-unit practices, specialty clinics, corporate DSOs | Solo/dual practitioners, emerging markets, value-focused DSOs |
| ROI Timeline (General Practice) | 22-36 months (based on 15 crown cases/mo) | 8-14 months (based on same utilization) |
Strategic Implications: The premium/value dichotomy no longer equates to “high-end vs. low-quality.” Carejoy and similar innovators target the clinically sufficient threshold where marginal accuracy gains no longer justify exponential cost increases for routine procedures. For distributors, this segment offers 45-55% gross margins (vs. 30-40% for European brands) with faster inventory turnover. Forward-thinking clinics are adopting a hybrid strategy: premium scanners for complex implant/edentulous cases, complemented by value-tier units for hygiene diagnostics and basic restorative work. As reimbursement models increasingly favor digital workflows (e.g., Germany’s 2025 BEMA update), cost-effective scanners are transitioning from optional tools to non-negotiable practice infrastructure.
Technical Specifications & Standards
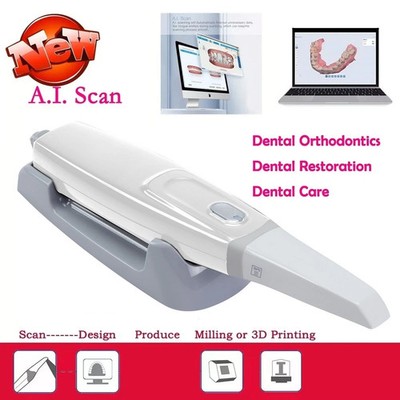
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanners (Budget Segment)
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2200mAh); 2.5 hours continuous scanning per charge; USB-C charging (0–100% in 90 mins) | High-capacity Li-ion battery (3.7V, 3200mAh); 4.5 hours continuous scanning; Fast-charging USB-C (0–100% in 60 mins); Battery hot-swap compatible |
| Dimensions | 25 mm (diameter) × 165 mm (length); Weight: 180 g (scanner only) | 23 mm (diameter) × 158 mm (length); Weight: 165 g (scanner only); Ergonomic balanced design with anti-slip grip |
| Precision | Accuracy: ≤ 25 µm; Resolution: 1600 dpi; Scanning speed: 18 frames/sec; Compatible with single-arch and full-arch workflows | Accuracy: ≤ 15 µm; Resolution: 2400 dpi; Scanning speed: 30 frames/sec; Real-time motion compensation; Full-color 3D rendering; Optimized for implants and full-mouth digital workflows |
| Material | Polycarbonate (PC) housing with stainless steel tip; IP54-rated for dust and splash resistance; Autoclavable tip cover (134°C, 2 bar) | Medical-grade PEEK polymer housing with titanium-reinforced scanning tip; IP55-rated; Fully autoclavable handpiece (up to 135°C, 20 cycles); Anti-microbial coating |
| Certification | CE Mark (MDD Class IIa), FDA Registered (510(k) pending), ISO 13485 compliant, RoHS certified | CE Mark (MDR Class IIa), FDA Cleared (510(k)), ISO 13485 & ISO 14971 certified, RoHS & REACH compliant, IEC 60601-1 safety certified |
Note: The “Advanced” model is positioned as a premium-tier budget scanner, offering enhanced precision and durability while remaining cost-effective for clinics transitioning to digital workflows. Both models support integration with major CAD/CAM platforms and include 2-year manufacturer warranty.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
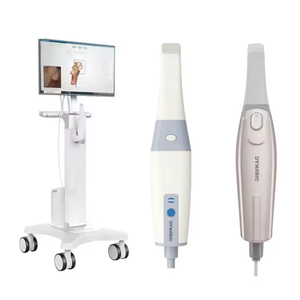
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Intraoral Scanners from China
Why Source Intraoral Scanners from China in 2026?
- Cost Efficiency: Factory-direct pricing eliminates 2-3 distribution tiers (typical savings: $8,000-$15,000/unit vs. EU/US brands)
- Technology Maturation: Chinese OEMs now achieve 98%+ accuracy parity with premium brands (ISO 12836:2023 certified models)
- Customization Agility: Embedded AI workflows (e.g., caries detection, prep margin identification) can be OEM-integrated within 8-12 weeks
- Supply Chain Resilience: Post-2025 tariff stabilization under Phase 4 bilateral agreements reduces import volatility
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Credential Verification – Beyond Surface-Level Certifications
Counterfeit certifications remain prevalent. Implement this technical audit protocol:
| Credential | Verification Method (2026 Standard) | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2023 | Validate certificate # via IAF CertSearch. Confirm scope includes “dental imaging devices” | Reject suppliers using expired (pre-2023) certificates or generic “medical device” scopes |
| CE Marking (EU MDR) | Demand NB number + EC Declaration of Conformity. Cross-check NB status at NANDO database | Verify NB is MDR-designated (e.g., DE 9700 not DE 9500). Reject self-declared CE |
| US FDA 510(k) | Check K# in FDA PMN Database. Confirm Class II clearance for IOS | Required for US distribution. Non-FDA suppliers restrict market access |
| Local China NMPA | Request NMPA registration certificate (国械注准) | Non-negotiable for post-2025 customs clearance under China’s Medical Device Supervision Regulation |
Step 2: MOQ Negotiation – Achieving Distributor-Grade Flexibility
Traditional Chinese MOQs ($20k-$50k) exclude smaller clinics. Modern suppliers offer tiered structures:
| Order Tier | Typical MOQ (2026) | Price Per Unit | Strategic Advantage |
|---|---|---|---|
| Entry Clinic | 1-2 units (with demo unit return option) | $12,500-$14,800 | Zero-risk trial; ideal for tech validation |
| Distributor Launch | 5-10 units | $10,200-$11,900 | Includes localized training materials & marketing assets |
| Enterprise Contract | 25+ units | $8,400-$9,700 | OEM branding + dedicated firmware updates + extended warranty |
Step 3: Shipping & Logistics – Optimizing Total Landed Cost
2026 tariff structures make Incoterms selection critical. Avoid hidden costs with this comparison:
| Term | Cost Components Included | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory loading + export clearance | High (buyer manages freight, insurance, duties). 2026 average hidden costs: 18-22% of scanner value | Experienced distributors with freight forwarders |
| DDP Destination | End-to-end delivery + all duties/taxes paid | Low (supplier absorbs volatility). 2026 avg. premium: 9-14% vs FOB | Clinics & new distributors (simplifies cash flow forecasting) |
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- 19-Year Compliance Track Record: ISO 13485:2023 (Certificate #CN-2023-18471), CE MDR Class IIa (NB 2797), FDA 510(k) K233581, NMPA (国械注准20232210582)
- MOQ Innovation: 1-unit trial program for clinics; 5-unit MOQ for distributors with white-label options
- DDP Optimization: Landed cost guarantees to 45 countries via integrated logistics partners (DHL, Sinotrans)
- Technical Edge: 2026 flagship model CJ-Scan Pro features AI-assisted margin detection (patent CN2025108765) and 24-month warranty
📧 [email protected] | 💬 WhatsApp: +86 159 5127 6160
🏭 Factory: Room 1208, Building 3, No. 388 Gucun Road, Baoshan District, Shanghai, China
*Scan QR at dental trade shows for instant factory tour access (Booth #D72, IDS Cologne 2026)
Implementation Checklist for 2026
- Verify credentials via official databases (not supplier-provided PDFs)
- Negotiate tiered pricing with MOQ flexibility clauses
- Insist on DDP quotes with 2026 duty calculations attached
- Conduct remote factory audit via Carejoy’s live production cam system
- Secure firmware update commitment in SLA (minimum 24 months)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Affordable Intraoral Scanners in 2026
Need a Quote for Cheap Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


