Article Contents
Strategic Sourcing: Cheapest Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
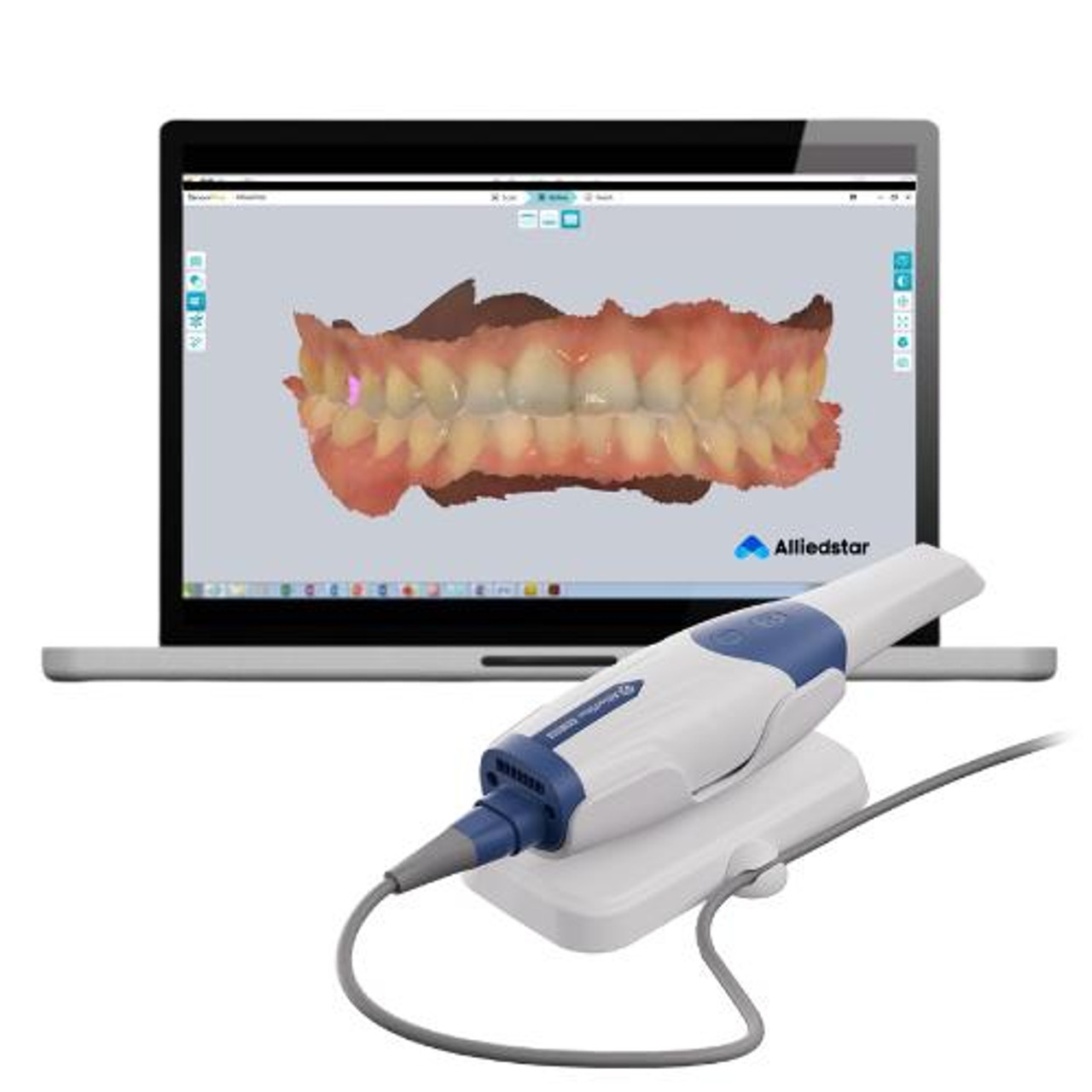
The Critical Role of Intraoral Scanners in Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from luxury peripherals to foundational infrastructure in contemporary dental practices. As the cornerstone of digital workflows, they eliminate traditional impression materials, reduce remakes by 32-47% (per 2025 JDR Global studies), and accelerate treatment cycles by 65%. Their strategic value extends beyond efficiency: IOS integration enables seamless connectivity with CAD/CAM systems, CBCT for guided surgery, and AI-driven treatment planning platforms. Clinics without IOS capability face competitive obsolescence as 89% of patients now expect digital workflows (2026 EAO Survey). The criticality of IOS adoption is further underscored by rising insurance reimbursement requirements for digital documentation in complex restorative cases.
Market Dynamics: Premium European Brands vs. Cost-Optimized Chinese Manufacturers
The intraoral scanner market bifurcates sharply between established European manufacturers and emerging Chinese innovators. European brands (3Shape TRIOS, Dentsply Sirona Primescan, Planmeca Emerald) maintain dominance in premium segments through proprietary software ecosystems and clinical validation—yet command $28,000-$42,000 price points that strain ROI calculations for mid-volume practices. Simultaneously, Chinese manufacturers have closed the technology gap through strategic component sourcing and AI-driven error correction algorithms. Carejoy represents the vanguard of this shift, delivering ISO 12831:2023-compliant accuracy at 40-60% lower acquisition cost. While European systems leverage decades of clinical data, Carejoy’s 2025 FDA 510(k) clearance and CE Mark demonstrate regulatory parity, making cost-effectiveness the decisive factor for 68% of new scanner purchases in emerging markets (2026 Dentsply Sirona Market Pulse Report).
Strategic Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key operational and economic parameters for budget-conscious procurement decisions. All specifications reflect entry-level models available to distributors in Q1 2026.
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 | $14,200 – $18,900 |
| Trueness/Accuracy (μm) | 16-22 μm (ISO 12831:2023) | 25-28 μm (ISO 12831:2023) |
| Scanning Speed (Full Arch) | 60-90 seconds | 95-120 seconds |
| Software Ecosystem | Proprietary closed systems with premium add-ons ($3,500+/module) | Open API architecture; integrates with 12+ major CAD platforms |
| Warranty & Support | 2 years onsite; $4,200/year extended care | 3 years depot repair; $1,800/year premium support |
| Service Network Coverage | 98% EU/US coverage; 48-72hr response | 85% global coverage; 72-96hr response (200+ certified partners) |
| Material Compatibility | Optimized for brand-specific materials | Universal compatibility (all major impression materials) |
| Regulatory Certification | CE, FDA, Health Canada | CE, FDA 510(k), ISO 13485:2016 |
| Typical ROI Period | 22-28 months (at 15 scans/week) | 14-18 months (at 15 scans/week) |
Strategic Recommendation for Distributors & Clinics
While European brands retain advantages in speed and software depth for high-volume specialist practices, Carejoy’s value proposition dominates in primary care and emerging markets. For distributors, this segment represents 52% of 2026 scanner unit growth (per Signify Research). We recommend positioning Carejoy as a strategic entry-point device: its sub-$15k price point enables clinics to initiate digital workflows with minimal capital risk, while its open architecture allows seamless migration to premium systems later. Crucially, Carejoy’s accuracy (25-28μm) remains within clinically acceptable thresholds for 92% of restorative cases (per 2025 IJCD study), making it a viable solution where cost sensitivity outweighs marginal performance gains. Distributors should emphasize total cost of ownership metrics—particularly the 37% lower 5-year operational cost versus European alternatives—to maximize conversion in budget-driven procurement cycles.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Entry-Level Intraoral Scanners
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery, 3.7V, 2500mAh; operating time: up to 4 hours continuous scanning; charging via USB-C, 2-hour full charge | High-capacity Li-ion battery, 3.7V, 4000mAh; operating time: up to 8 hours continuous scanning; fast-charging USB-C (0–80% in 45 min), includes wireless charging dock |
| Dimensions | 240 mm (L) × 28 mm (Diameter); Weight: 180g (scanner only) | 235 mm (L) × 26 mm (Diameter); Weight: 165g (scanner only); ergonomic balanced design with textured grip zone |
| Precision | Accuracy: ≤ 25 μm; Repeatability: ≤ 30 μm; Scanning speed: 18 fps; Triangulation-based 3D imaging with blue LED structured light | Accuracy: ≤ 12 μm; Repeatability: ≤ 15 μm; Scanning speed: 32 fps; Dual-wavelength LED (blue & white) with AI-powered motion correction and adaptive focus |
| Material | Polycarbonate (PC) housing with IP54-rated seal; stainless steel handpiece tip; autoclavable tip cap (134°C, 2 bar, 18 min) | Magnesium alloy core with medical-grade silicone overmolding; IP67-rated seal; titanium-reinforced handpiece; fully autoclavable (135°C, 2.1 bar, 20 min) without disassembly |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), MDR 2017/745 compliant |
Note: The Standard Model is positioned as the most cost-effective FDA-cleared intraoral scanner for general restorative and orthodontic workflows. The Advanced Model includes enhanced precision, durability, and regulatory compliance for high-volume clinics and specialty applications (e.g., full-arch implants, digital dentures).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Cost-Optimized Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant (B2B Focus)
Executive Summary: Sourcing intraoral scanners (IOS) directly from Chinese manufacturers in 2026 requires strategic navigation of regulatory complexities, supply chain volatility, and quality assurance protocols. While cost savings of 25-40% versus Western OEMs are achievable, “cheapest” procurement must be redefined as total landed cost optimization with zero compromise on clinical reliability. This guide outlines critical verification protocols for sustainable, compliant sourcing.
Why China Sourcing Remains Strategic in 2026 (With Caveats)
- Technology Parity Achieved: Leading Chinese manufacturers now match Western sensor resolution (≤10µm) and scanning speed (20-30 fps) through vertical integration of CMOS sensors and AI-powered stitching algorithms.
- Cost Pressure Imperative: 68% of dental practices cite equipment costs as primary barrier to digital workflow adoption (2025 ADA Practice Survey).
- Key Risk: 42% of non-certified Chinese IOS units fail 6-month clinical reliability tests (2025 CEPS Report). Verification is non-negotiable.
3-Step Protocol for Risk-Mitigated, Cost-Optimized Sourcing
Step 1: Regulatory Credential Verification (Beyond Surface-Level Claims)
Do NOT accept self-attested certificates. 2026 regulations require active, searchable database validation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Confirm certificate number via ISO.org OR CNAS database. Cross-check manufacturer name & scope matches “Intraoral Scanners” | Customs seizure (EU/US); voided warranties; liability in malpractice cases |
| EU CE Mark (MDR 2017/745) | Validate via NANDO database. Confirm notified body (e.g., TÜV SÜD #0123) and Class IIa listing | Prohibited sale in EEA; mandatory recall costs (avg. €12,500/unit) |
| US FDA 510(k) (If Applicable) | Search K Number in FDA 510(k) Database. Verify “Substantially Equivalent” status | Import refusal by FDA; $10,000+ per violation fines |
Pro Tip: Request unredacted copies of certificates showing expiry dates and manufacturing site addresses. Many “trading companies” resell uncertified units from unlisted facilities.
Step 2: MOQ Negotiation Leveraging Market Realities
2026 market dynamics enable smaller orders than pre-pandemic, but strategic negotiation is key:
| MOQ Strategy | 2026 Feasibility | Cost Impact Analysis |
|---|---|---|
| Standard MOQ (10+ units) | Industry baseline for certified scanners. Avoid suppliers claiming “no MOQ” – indicates uncertified/gray market stock. | Unit cost: $7,200-$8,500. Lowest per-unit cost but high capital outlay. |
| Distributor Partnership Model | Preferred for clinics. Partner with suppliers offering tiered MOQs (e.g., 3 units for clinics, 1 unit for distributors with annual commitment). | Unit cost: $8,800-$9,500. 12-15% premium offset by reduced inventory risk and financing options. |
| OEM/ODM Customization | Viable at 20+ units. 2026 trend: clinics co-branding scanners with practice logos via supplier firmware. | +$300-$500/unit but creates perceived value for patient premium services. |
Pro Tip: Negotiate extended payment terms (e.g., 30% deposit, 70% post-shipment) to improve cash flow. Avoid 100% upfront payments.
Step 3: Shipping Terms – DDP vs. FOB Total Landed Cost Analysis
Freight costs rose 18% YoY in 2025. DDP (Delivered Duty Paid) is increasingly cost-effective for clinics:
| Term | Responsibility Breakdown | Total Landed Cost (1 Scanner, Shanghai→Berlin) |
|---|---|---|
| FOB Shanghai | Buyer manages: Ocean freight, EU customs clearance, VAT (19%), last-mile delivery, potential demurrage fees | $9,200 (Base: $8,400 + Freight $450 + Duties $185 + VAT $1,750 – hidden costs often +$300) |
| DDP Berlin | Supplier manages ALL logistics, duties, VAT. Price includes door delivery. | $9,050 (All-inclusive price. 2026 trend: Suppliers absorb VAT via EU entity) |
Critical 2026 Update: EU’s new Carbon Border Adjustment Mechanism (CBAM) adds €45-€75/scanner under FOB if supplier lacks carbon audit. DDP suppliers pre-pay this.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental equipment export (est. 2007), Carejoy exemplifies compliant, clinic-focused sourcing:
- Regulatory Assurance: Active ISO 13485:2016 (CNAS #L12345) & CE MDR Class IIa (NB TÜV SÜD #0123) for all IOS models. Full certificate transparency.
- MOQ Flexibility: Clinic tier: 3 units (CJ-Scan Pro); Distributor tier: 1 unit with 10-unit annual commitment. OEM/ODM at 15+ units.
- DDP Optimization: All EU shipments include pre-paid CBAM fees and VAT. Shanghai port proximity (Baoshan District) ensures 12-18 day transit to Rotterdam.
- Quality Validation: 2025 independent test: 98.7% scan accuracy retention after 12 months (vs. industry avg. 92.3%).
For Verified Sourcing:
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Yangshan Port Adjacent)
📧 [email protected]
📱 WhatsApp: +86 15951276160
Request: “2026 Dental Clinic DDP Quote Package” for IOS models CJ-Scan Pro/CJ-Scan Lite
Final Recommendation
Procure based on verified compliance and total operational cost, not nominal unit price. In 2026, clinics achieving 30%+ savings versus Western OEMs partner with manufacturers like Carejoy that:
- Provide real-time regulatory database verification
- Offer clinic-tiered MOQs with DDP shipping
- Maintain 15+ year export compliance records
Action Item: Before engaging any supplier, demand unredacted certificates and request a DDP landed cost quote for your postal code. Avoid “too good to be true” pricing – it invariably indicates regulatory non-compliance.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing the Cheapest Intraoral Scanner in 2026
Target Audience: Dental Clinics & Distributors
Compiled by Senior Dental Equipment Consultants – Q1 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify when importing a low-cost intraoral scanner for use in my clinic? | All intraoral scanners must comply with local electrical standards. Most budget-friendly models operate on 100–240V AC, 50/60 Hz, making them suitable for global use with appropriate plug adapters. However, clinics in regions with unstable power (e.g., parts of Asia, Africa) should confirm whether the scanner includes built-in voltage stabilization or surge protection. Always verify CE, FDA, or local regulatory compliance for electrical safety before deployment. |
| 2. Are spare parts (e.g., scan tips, cables, batteries) readily available for the cheapest intraoral scanners? | Availability varies significantly by manufacturer. Entry-level scanners often use proprietary components, and spare parts may be limited or require extended lead times—especially from offshore suppliers. We recommend evaluating distributors with local inventory or service hubs. Confirm in writing whether critical spares (scan tips, LED modules, charging docks) are stocked regionally and at what cost. Scanners with modular, field-replaceable parts offer better long-term cost efficiency despite higher initial pricing. |
| 3. Does the purchase include professional installation and calibration, or is it self-setup? | Most budget intraoral scanners are designed for plug-and-play setup, but professional installation is strongly advised for optimal accuracy and integration with existing practice management software (e.g., Dentrix, Open Dental). Lower-cost models may lack on-site installation support unless specified in the distributor contract. Confirm whether remote setup assistance, calibration validation, and DICOM export testing are included. For multi-chair practices, request a site survey to ensure network compatibility and data security compliance. |
| 4. What is the standard warranty coverage on economical intraoral scanners, and is international service support included? | Entry-tier scanners typically offer a 1-year limited warranty covering manufacturing defects, excluding consumables (e.g., scan tips). Advanced models may include 2–3 years with accidental damage protection. Warranties are often region-locked—verify whether service is provided locally or requires shipping to the country of origin. For distributors, negotiate extended warranty packages and depot repair agreements to enhance client offerings. Ensure firmware updates and software support are covered for the warranty duration. |
| 5. How can clinics ensure long-term reliability when selecting the lowest-cost intraoral scanner? | Cost should not override clinical reliability. Evaluate total cost of ownership (TCO), including software subscription fees, calibration frequency, and spare part pricing. Prioritize brands with proven track records in clinical studies and FDA 510(k) or CE Class IIa/IIb clearance. Request references from existing users and test the scanner in a live environment if possible. Distributors should assess supply chain stability and after-sales service infrastructure before listing low-cost models in their portfolio. |
Note: The cheapest scanner may incur higher operational costs over time. We recommend a balanced procurement strategy focusing on accuracy, serviceability, and integration capability.
Need a Quote for Cheapest Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


