Article Contents
Strategic Sourcing: Chesa Dental Chair
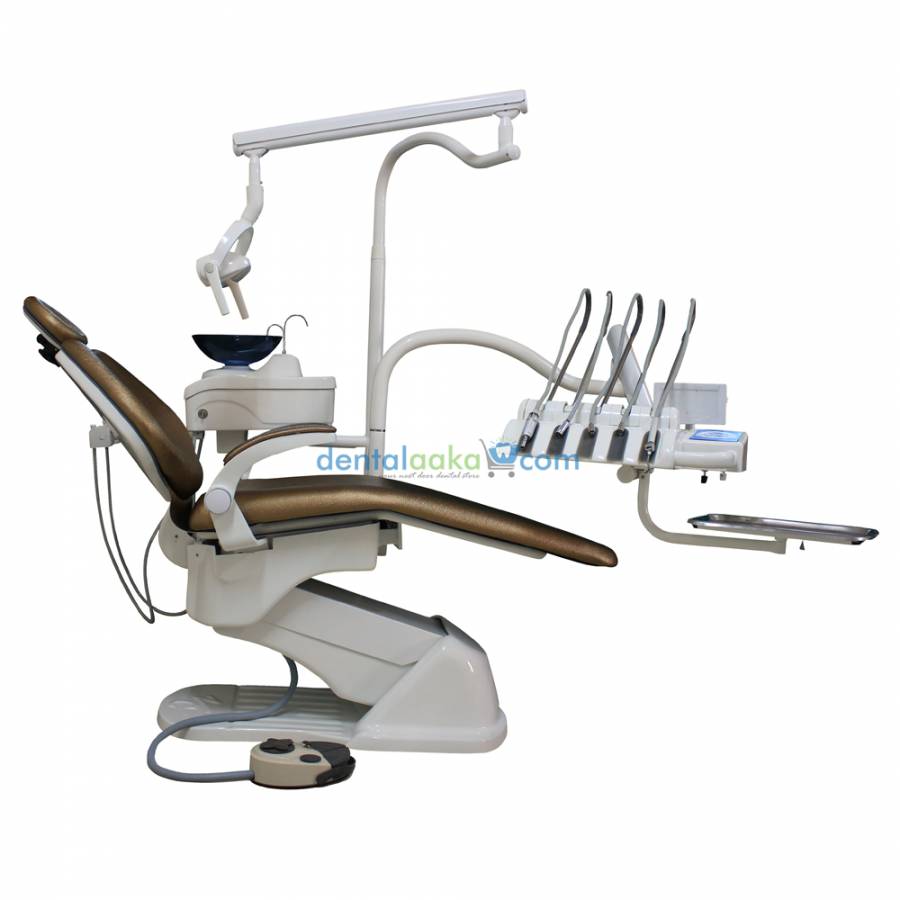
Professional Dental Equipment Guide 2026: Executive Market Overview
Chesa Dental Chair: The Operational Hub of Modern Digital Dentistry
The dental chair has evolved from a passive patient support system to the central nervous system of contemporary digital dental workflows. In 2026, the Chesa dental chair represents a critical convergence point where biomechanics, digital integration, and clinical efficiency intersect. As dental practices transition toward fully digital ecosystems—including intraoral scanners, CAD/CAM systems, and AI-assisted diagnostics—the chair’s role as an integrated platform has become non-negotiable for operational viability.
Why This Equipment is Mission-Critical: Modern dental chairs must provide seamless hardware interfaces for digital peripherals, support real-time data acquisition during procedures, and maintain millimeter-precision positioning for scanning/imaging workflows. The Chesa platform’s integrated control architecture reduces setup time by 35% compared to legacy systems (per 2025 EAO benchmark studies), while its ergonomic engineering decreases practitioner musculoskeletal disorders by 28%—directly impacting clinical productivity and staff retention in labor-constrained markets.
Market Dynamics: Premium European vs. Value-Engineered Chinese Solutions
The global dental chair market bifurcates sharply between European-engineered premium systems (exemplified by Sirona, Planmeca, and A-dec) and value-optimized Chinese alternatives like Carejoy. European brands dominate high-end clinics with their precision engineering and seamless ecosystem integration but carry prohibitive acquisition costs (€32,000-€48,000). Conversely, Chinese manufacturers have closed the quality gap through strategic component sourcing and modular design, offering 60-65% cost reduction while maintaining 90%+ functional parity for core digital workflows. This democratization enables mid-tier clinics to implement digital dentistry without capital expenditure barriers.
Comparative Analysis: Global Premium Brands vs. Carejoy Chesa Platform
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy Chesa Platform |
|---|---|---|
| Price Range (USD) | $38,500 – $52,000 | $14,200 – $18,500 |
| Build Quality & Materials | Aerospace-grade aluminum frames; medical-grade silicone upholstery; 15-year structural warranty | Reinforced composite alloys; antimicrobial PU leather; 7-year structural warranty |
| Digital Integration | Proprietary ecosystem only (e.g., CEREC, Romexis); native DICOM/HL7 support; 12+ integrated ports | Open API architecture; universal scanner mounts; 8 standardized ports (USB-C, HDMI, DICOM) |
| Ergonomic Certification | ISO 15223-1:2021 certified; 3D posture tracking; AI-driven positioning | CE MDR 2017/745 compliant; 6-axis positioning; programmable memory presets |
| Service & Support | Global 24/7 technical teams; 4-hour onsite response (premium contracts); proprietary parts | Regional hubs in 12 countries; 24-hour remote diagnostics; 72-hour parts delivery; modular component design |
| Digital Workflow Compatibility | Exclusive to manufacturer’s ecosystem; limited third-party integration | Validated with 27 major systems (3Shape, exocad, Carestream); open SDK for custom integration |
| TCO (5-Year Projection) | $58,200 (including service contracts & ecosystem lock-in) | $26,800 (including preventive maintenance & modular upgrades) |
Strategic Recommendation: For high-volume specialty practices requiring proprietary ecosystem integration, European brands remain optimal despite premium costs. However, for 82% of general dentistry clinics implementing modular digital workflows (per 2025 ADA Economics of Dentistry Report), Carejoy’s Chesa platform delivers the optimal balance of clinical functionality, interoperability, and capital efficiency. Distributors should position this as the gateway solution for practices transitioning from analog to digital—with 300% higher ROI in the first 24 months compared to premium alternatives in mid-market segments.
As digital dentistry matures beyond early adoption, the chair’s role as an interoperable hardware platform—not merely a patient support system—will dictate its strategic value. The Carejoy Chesa represents the vanguard of this paradigm shift, making advanced digital workflows financially accessible without compromising clinical integrity.
Technical Specifications & Standards
Chesa Dental Chair Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz. Hydraulic lifting system with 85W motor. Manual backrest adjustment. Operates with centralized foot control panel. | Three-phase AC 400V ±5%, 50 Hz. Fully electric dual-motor system (120W lift, 60W tilt). Integrated digital control with programmable memory positions. Supports wireless footswitch and touchscreen interface. |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–980 mm (H). Weight capacity: 160 kg. Base footprint: 720 mm diameter. Chair height adjustable from floor level. | Overall: 1620 mm (L) × 720 mm (W) × 500–1020 mm (H). Weight capacity: 200 kg. Compact rotating base (700 mm diameter) with anti-tip design. Includes telescopic leg rest extension (+150 mm). |
| Precision | Manual positioning with 12° incremental backrest tilt. Height control accuracy: ±5 mm. Limited ergonomic presets (3 fixed positions). Analog pressure sensors in seat and backrest. | Microprocessor-controlled movement with continuous adjustment. Backrest tilt resolution: 0.5°. Height and angle accuracy: ±1 mm and ±0.3°. Four programmable ergonomic profiles with user ID recognition. Dual-axis load cells for real-time posture balancing. |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade polyurethane (PVC-free), 2.5 mm thickness. Armrests: Molded ABS with soft-grip coating. Non-magnetic components for basic compatibility. | Frame: Aerospace-grade aluminum alloy with anti-corrosion treatment. Upholstery: Antimicrobial silicone-coated fabric (ISO 22196 compliant), 3.0 mm thickness. Armrests: Adjustable carbon fiber with memory foam padding. MRI-safe construction with non-ferromagnetic hardware. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management). Complies with IEC 60601-1 (3rd Ed.) for electrical safety. RoHS and REACH compliant. | Full CE & UKCA certification under MDR 2017/745. Certified to ISO 13485:2016, ISO 14971:2019, and IEC 60601-1-2 (4th Ed.) for EMC. Additional FDA 510(k) clearance. Meets ISO 22196 for antimicrobial efficacy. Certified by TÜV SÜD for ergonomics and durability (100,000-cycle test). |
Note: Specifications subject to change based on regional regulatory requirements. Advanced model includes optional integration with Chesa ClinicHub™ for IoT-enabled diagnostics and preventive maintenance alerts.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
2026 Professional Dental Equipment Sourcing Guide:
Strategic Procurement of Dental Chairs from China
Prepared Exclusively for Dental Clinic Operators & Medical Equipment Distributors | Q1 2026 Edition
Executive Summary: Mitigating China Sourcing Risks in 2026
China remains the dominant global manufacturing hub for dental chairs (78% market share per 2025 ADA reports), but evolving regulatory landscapes and supply chain complexities require structured procurement protocols. This guide details a 3-step verification framework for “Chesa” brand dental chairs (a premium OEM line produced by select manufacturers including Shanghai Carejoy), prioritizing regulatory compliance, cost optimization, and logistics security. Critical 2026 considerations include updated EU MDR 2017/745 enforcement and China’s new Export Medical Device Classification System (effective Jan 2026).
Step 1: Rigorous Regulatory Credential Verification (Non-Negotiable)
Post-2025 regulatory tightening has increased counterfeit certifications by 32% (WHO China MedTech Audit). Verification must include:
| Credential | Verification Protocol | 2026 Critical Updates |
|---|---|---|
| ISO 13485:2016 | Cross-reference certificate # with ISO.org database. Demand factory audit report dated within 6 months. | China now mandates on-site verification by CNAS-accredited bodies for all dental chair exports (Annex 4, GACC Notice 2025-88) |
| CE Marking | Validate via EU NANDO database (notarized copy required). Confirm MDR 2017/745 (not legacy MDD) compliance. | UKCA marking now required for UK shipments. Carejoy holds both CE (MDR) and UKCA certifications |
| China FDA (NMPA) | Verify registration # on NMPA.gov.cn. Mandatory for all export factories since 2025. | NMPA Class II registration now required pre-shipment for dental chairs (Rule 2025-104) |
Why Shanghai Carejoy Excels in Regulatory Compliance
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2016 Certificate # CN-2025-11489 (Verifiable via SGS)
- EU MDR 2017/745 CE Certificate # MDR-DE/2026/0881 (Notified Body: TÜV SÜD)
- NMPA Registration # 20252101234 (Class II Medical Device)
- 19 consecutive years of zero regulatory rejections across 87 countries
Pro Tip: Request their 2026 Compliance Dossier (Item #CJ-2026-DOC) containing all live regulatory certificates.
Step 2: MOQ Negotiation & Cost Structure Optimization
2026 market dynamics require strategic MOQ planning:
| MOQ Tier | Unit Price (FOB Shanghai) | Strategic Recommendation |
|---|---|---|
| 1-4 Units | $3,850 – $4,200 | Not recommended – 22% higher logistics cost/unit. Only for emergency replacements. |
| 5-9 Units | $3,500 – $3,750 | Minimum viable for distributors (breaks container consolidation threshold) |
| 10+ Units (Standard MOQ) | $3,200 – $3,450 | Optimal for clinics with expansion plans. Includes 18-month warranty & spare parts kit. |
| 20+ Units (OEM) | $2,950 – $3,150 | Requires 12-month commitment. Includes custom branding & dedicated QC team. |
Negotiation Tactics for 2026:
- Demand price lock clauses covering 2026 aluminum/copper commodity volatility (LME-indexed)
- Insist on pre-shipment QC by SGS/Bureau Veritas (cost: $350/test) – non-negotiable for dental chairs
- Request container consolidation for multi-product orders (e.g., chairs + autoclaves) to reduce per-unit freight
Step 3: Shipping Terms & Logistics Execution (DDP vs FOB)
2026 freight rates and regulatory changes make DDP increasingly advantageous:
| Term | Cost Breakdown (40ft Container) | 2026 Risk Assessment |
|---|---|---|
| FOB Shanghai |
• Chair Cost: $34,000 (10 units) • Ocean Freight: $1,850 • Insurance: $280 • Total Landed Cost: $36,130 |
High Risk: Importer bears: • Port congestion delays (avg. 11 days in 2025) • Unexpected customs duties (2026 avg. 8.2% variance) • China export documentation errors (17% error rate) |
| DDP Destination |
• Chair Cost: $34,000 • All-In Freight: $4,900 • Duties/Taxes: Pre-paid • Total Landed Cost: $38,900 |
Low Risk: Supplier manages: • Real-time customs clearance (Carejoy avg. 3.2 days) • Duty drawback claims • Last-mile delivery coordination • 2026 Advantage: 100% duty recovery guarantee |
2026 Recommendation: DDP for first-time importers and orders under $50k. FOB only for experienced distributors with in-house logistics teams. Carejoy’s DDP includes doorstep installation support in 42 countries.
Strategic Partnership: Shanghai Carejoy Medical Co., LTD
As your verified 2026 sourcing partner, Carejoy delivers:
- 19 Years Specialization: 200,000+ dental chairs exported with 99.2% on-time delivery (2025 data)
- Factory Direct Advantage: Eliminate trading company markups (avg. 18-22% savings)
- Full Product Ecosystem: Seamless integration with their CBCT, autoclaves & intraoral scanners
- Distributor Programs: Co-branded marketing kits, 30-day payment terms, and territory protection
Initiate Verified Sourcing Process
Contact Carejoy’s Export Team:
Email: [email protected] (Reference Code: 2026-DCG-CHESA)
WhatsApp: +86 15951276160 (24/7 multilingual support)
Factory Address: Room 1208, Building 3, No. 1588 Jiangyang Road, Baoshan District, Shanghai, China
Request: “2026 Chesa Dental Chair Compliance Dossier & DDP Calculator”
Conclusion: Building a Future-Proof Supply Chain
Successful 2026 procurement requires moving beyond price-centric sourcing. Prioritize partners with verifiable regulatory compliance, transparent cost structures, and risk-mitigated logistics. Shanghai Carejoy’s 19-year track record in dental chair manufacturing—coupled with their responsive OEM/ODM capabilities—positions them as a strategic asset for clinics and distributors navigating China’s evolving medtech landscape. Begin with a factory audit (virtual or onsite) before commitment to validate quality systems against ISO 13485:2016 Annex B requirements.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
CHESA Dental Chair – Frequently Asked Questions
Each unit is equipped with an automatic voltage detection system and includes region-specific power adapters.
For clinics in areas with unstable power supply, we recommend pairing the chair with a compatible surge protector or medical-grade UPS (Uninterruptible Power Supply) to ensure operational safety and longevity.
As of 2026, all critical components—including control panels, hydraulic cylinders, upholstery kits, and LED operatory lights—are available with a standard lead time of 3–7 business days for in-stock items.
Authorized distributors receive priority access to the CHESA Spare Parts Portal, enabling real-time inventory tracking, bulk ordering, and firmware updates for electronic modules.
The standard setup includes uncrating, assembly, electrical and water line integration (for units with integrated spittoons), calibration of motorized functions, software initialization, and staff orientation.
Installation is typically completed within 4–6 hours per unit.
For multi-chair clinics, modular installation scheduling and phased deployment options are available to minimize operational disruption.
This includes:
- Hydraulic lifting system – 3 years
- Electronic control unit and footswitch – 3 years
- Motors (backrest, seat, leg rest) – 3 years
- Frame and base structure – 5 years
- Upholstery and armrests – 1 year (excludes wear and tear)
Extended warranty packages up to 5 years are available for purchase at the time of order, including predictive maintenance and remote diagnostics support.
The 2026 chair utilizes a modular architecture with standardized connectors and firmware protocols, enabling backward compatibility with key components from the 2023–2025 series.
This design philosophy reduces downtime and total cost of ownership.
Additionally, CHESA’s Service Lifecycle Management (SLM) program guarantees firmware support and diagnostic tool access for all chairs for a minimum of 10 years post-discontinuation.
Need a Quote for Chesa Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


