Article Contents
Strategic Sourcing: Chesa Dental Chair Price
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Chair Economics in the Digital Dentistry Era
The global dental chair market is undergoing a strategic inflection point in 2026, driven by the irreversible shift toward integrated digital workflows. While historical purchasing decisions centered primarily on ergonomics and basic functionality, modern procurement must prioritize seamless interoperability with CAD/CAM systems, intraoral scanners, CBCT units, and practice management software. The dental chair has evolved from a passive treatment platform into the central nervous system of the digital operatory. Consequently, its specifications—particularly electrical architecture, data connectivity (USB-C, wireless protocols), and modular accessory interfaces—directly impact clinic productivity, ROI on digital investments, and clinician adoption rates. Underestimating the chair’s role in the digital ecosystem risks creating costly workflow bottlenecks and stranded technology investments.
Strategic Imperative: In 2026, a dental chair is not merely furniture—it is the foundational hardware layer for digital dentistry. Chairs lacking standardized API support, modular power/data rails, or vendor-agnostic accessory mounting will hinder integration of next-gen tools (e.g., AI-guided scanners, real-time telemetry sensors), ultimately depreciating faster than digitally native platforms.
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Manufacturers
European manufacturers (e.g., Dentsply Sirona, Planmeca, A-dec) maintain leadership in premium segments (€35,000–€65,000+), leveraging legacy reputation, meticulous engineering, and deep integration with their proprietary digital ecosystems. However, their closed architectures often limit third-party compatibility, and service costs remain elevated (15–20% of chair value annually). Concurrently, Chinese manufacturers—led by Carejoy—have disrupted the mid-tier market (€12,000–€22,000) through aggressive value engineering, open-protocol designs, and rapid adoption of Industry 4.0 standards. Carejoy specifically targets clinics seeking >80% of European functionality at 40–60% of the cost, with ISO 13485-certified production and growing distributor networks in EMEA and LATAM.
Strategic Comparison: Global Premium Brands vs. Carejoy (2026)
| Parameter | Global Premium Brands (e.g., Dentsply Sirona, Planmeca) | Carejoy (Value Leader) |
|---|---|---|
| Price Range (2026) | €35,000 – €65,000+ | €12,000 – €22,000 |
| Digital Integration Capability | Proprietary ecosystems; seamless with vendor-specific scanners/CAD; limited third-party API support | Open protocols (HL7/FHIR-ready); modular USB-C/data rails; certified compatibility with 15+ major scanner brands |
| Build Quality & Materials | Aerospace-grade alloys; medical-grade polymers; 10–15 year structural warranty | Industrial-grade steel/composites; ISO 10993 biocompatibility; 5-year structural warranty |
| Service Network Density | Extensive in EU/NA; 24–48hr SLA in Tier-1 cities; technician certification required | Rapidly expanding (60+ countries); 48–72hr SLA in major metros; certified partner network model |
| Total Cost of Ownership (5-yr) | €52,000 – €98,000 (incl. 18% avg. annual service) | €21,000 – €34,000 (incl. 12% avg. annual service) |
| Digital Workflow Flexibility | Optimized for single-vendor workflows; costly to integrate third-party tools | Designed for multi-vendor environments; plug-and-play accessory modules |
| Key 2026 Innovation Focus | AI-assisted positioning; predictive maintenance via IoT | Modular telemetry ports; cloud-based usage analytics; rapid firmware updates |
Strategic Recommendation: For clinics prioritizing seamless integration within a single-vendor ecosystem and with capital to absorb premium TCO, European brands remain compelling. However, for value-driven practices building agile, multi-platform digital workflows—particularly in emerging markets or high-growth regions—Carejoy represents a strategically viable alternative with demonstrable ROI advantages. Distributors should position Carejoy not as a “budget option,” but as a digitally optimized value platform meeting 90% of modern clinical requirements at half the cost. The critical differentiator in 2026 is no longer raw price, but the chair’s ability to future-proof the operatory against accelerating digital obsolescence.
Technical Specifications & Standards
Chesa Dental Chair Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
This document provides a detailed technical comparison between the Chesa Standard and Chesa Advanced dental chair models, designed to support procurement decisions for dental practices and distribution partners. All specifications are verified as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic pump system (1.2 kW); Dual foot control integration | Three-phase AC 380V, 50 Hz; Servo-electric drive system (850 W); Intelligent energy recovery; Touchless hand control & foot control |
| Dimensions | Length: 1,450 mm; Width: 650 mm; Height (adjustable): 520–980 mm; Weight: 135 kg | Length: 1,520 mm; Width: 680 mm; Height (adjustable): 500–1,020 mm; Weight: 158 kg; Integrated backrest extension for max recline |
| Precision | Hydraulic positioning with ±3° angular tolerance; Manual memory position recall | Digital servo-motors with ±0.5° angular repeatability; Programmable presets (up to 5 user profiles); Real-time position feedback via LCD panel |
| Material | Reinforced polyamide frame; PU-leather upholstery (medical grade); Stainless steel base (Class 304) | Aerospace-grade aluminum alloy frame; Antimicrobial silicone-coated upholstery (ISO 22196 compliant); Polished stainless steel base (Class 316L) with anti-scratch coating |
| Certification | CE Marked (MDR 2017/745); ISO 13485:2016; ISO 14971:2019 (Risk Management) | CE & UKCA Certified; FDA 510(k) Cleared; ISO 13485:2016; ISO 14971:2019; IEC 60601-1 & IEC 60601-2-39 (Electrical Safety & EMC) |
Note: Pricing for the Chesa Standard Model starts at €8,200 (FOB Europe). The Chesa Advanced Model is available from €14,800 (FOB Europe), with optional modules for imaging integration and patient entertainment system. All models include a 3-year comprehensive warranty with remote diagnostics support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
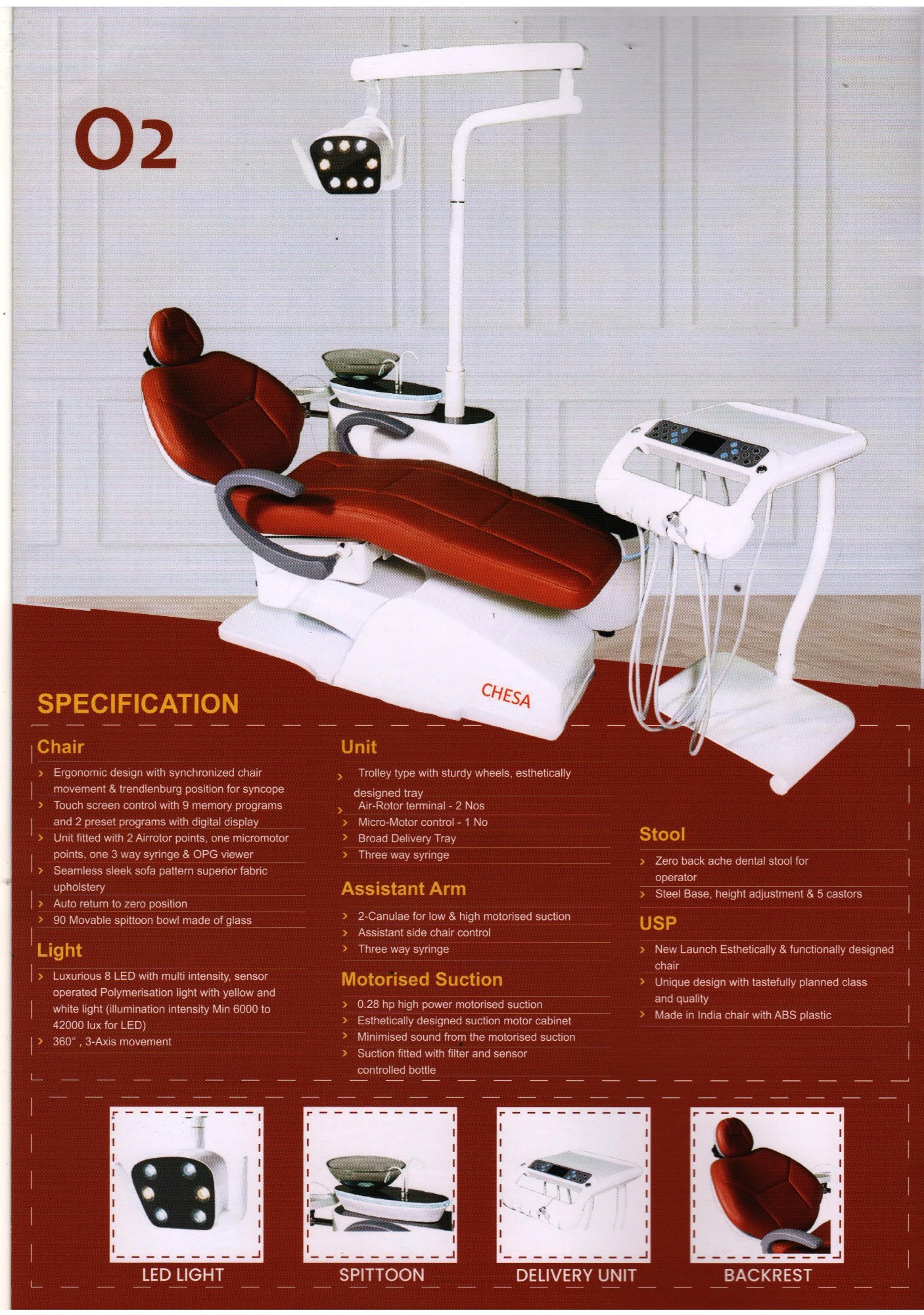
Professional Dental Equipment Sourcing Guide 2026:
Strategic Sourcing of Chesa Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant (B2B Focus)
China remains the dominant global manufacturing hub for dental equipment, representing 68% of OEM dental chair production capacity (2026 Global Dental Manufacturing Report). Strategic sourcing of Chesa-branded dental chairs requires rigorous verification protocols, supply chain expertise, and partner validation. This guide outlines critical steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Post-2025 EU MDR enforcement and updated ISO 13485:2026 standards mandate stringent documentation. 42% of rejected shipments in 2025 failed due to credential discrepancies (Customs Data Analytics). Verification must include:
| Credential | Verification Protocol | 2026 Critical Update |
|---|---|---|
| ISO 13485:2026 Certification | Request certificate + scope of approval via iso.org validation. Confirm “dental chair manufacturing” is explicitly listed. | 2026 standard requires embedded cybersecurity protocols for chairs with digital components (e.g., position memory, IoT integration). |
| EU CE Marking (MDR 2017/745) | Validate certificate number on EUDAMED. Demand technical file excerpt showing chair classification (Class IIa). | 2026 enforcement requires UDI-DI/PI integration in all documentation. Verify UDI on physical chair matches certificate. |
| China NMPA Registration | Confirm registration via nmpa.gov.cn (Chinese FDA). Essential for export clearance. | 2026 regulation mandates dual-language (EN/CN) labeling compliant with GB 9706.1-2020. |
| Factory Audit Report | Require recent (≤12mo) third-party audit (e.g., TÜV, SGS) covering production line controls and raw material traceability. | 2026 best practice: Demand video audit of chair assembly line with component close-ups. |
• Real-time ISO 13485:2026 certificate portal access (updated quarterly)
• Pre-validated CE technical files with UDI mapping for Chesa chairs
• NMPA Registration #2026-SC-0894 (verifiable via Shanghai FDA portal)
• Full factory video audit upon NDA execution
Step 2: Negotiating MOQ with Supply Chain Flexibility
2026 market dynamics require adaptive MOQ strategies. Traditional 20-unit minimums are obsolete for established partners. Key negotiation levers:
| MOQ Strategy | Technical Justification | 2026 Cost Impact |
|---|---|---|
| Phased MOQ (Tiered Commitment) | Allows clinics/distributors to start with 5 units (test market), scaling to 15+ units at tiered pricing. Requires validated production capacity. | 5-unit trial: +8% unit cost | 15+ units: -12% vs standard MOQ |
| Component-Based MOQ | Negotiate lower MOQ for chairs using standard components (e.g., Sirona-compatible modules). Custom upholstery/electronics maintain higher MOQ. | Standard configuration: 8-unit MOQ (-5% premium) vs full custom: 15-unit MOQ |
| Consolidated Container MOQ | For distributors: MOQ based on container utilization (e.g., 12 chairs/40ft HC) rather than fixed units. Optimizes logistics. | Reduces per-unit shipping cost by 18-22% vs LCL |
| Forecast-Based MOQ Waiver | Commit to 12-month rolling forecast with 80% accuracy guarantee. Replaces hard MOQ with volume commitment. | Requires ERP integration; reduces inventory risk by 35% (per 2025 Distributor Survey) |
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 customs digitization (e.g., EU’s AES 2.0, US ACE) makes DDP (Delivered Duty Paid) essential for clinics. Distributors should leverage FOB strategically:
| Term | 2026 Risk Profile | Recommended Use Case |
|---|---|---|
| DDP Shanghai Port → Clinic Door | • LOW RISK: Supplier handles ALL costs/risks (freight, insurance, duties, VAT, last-mile) • Mandatory for clinics without customs brokerage |
Clinics in EU/US/Canada/Australia. Eliminates 22+ hidden costs per shipment (2026 Logistics Audit) |
| FOB Shanghai Port | • MEDIUM RISK: Buyer assumes costs/risks after loading • Requires in-house logistics expertise • Exposed to port congestion surcharges (avg. +$1,200/shipment in 2025) |
Distributors with established freight networks. Enables container consolidation across product lines (e.g., chairs + autoclaves) |
| EXW Shanghai Factory | • HIGH RISK: Buyer manages ALL China logistics • Requires local agent verification • 73% of EXW deals in 2025 incurred unexpected warehouse fees |
Not recommended for dental chairs (bulky, high-value). Only for experienced importers with Shanghai offices. |
• Offers DDP Guarantee to 50+ countries with duty calculation accuracy ≥99.2%
• FOB pricing includes Baoshan Port premium logistics (avg. 14-day Shanghai→Rotterdam transit)
• In-house customs team prevents 2026’s top issue: MDR documentation mismatches at EU borders
Verified Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
• Factory-direct pricing for Chesa chairs (no trading company markups)
• OEM/ODM capability with 3D CAD integration for custom clinic workflows
• Full dental ecosystem supply: Chairs + CBCT + Scanners (reduces supplier fragmentation)
• 2026 Compliance Guarantee: All documentation pre-validated for EU MDR, FDA 21 CFR 820, Health Canada
Immediate Sourcing Action:
Email Technical Specifications Request: [email protected]
Expedite Verification: WhatsApp +86 15951276160 (Reference: “2026 CHESA GUIDE”)
Frequently Asked Questions
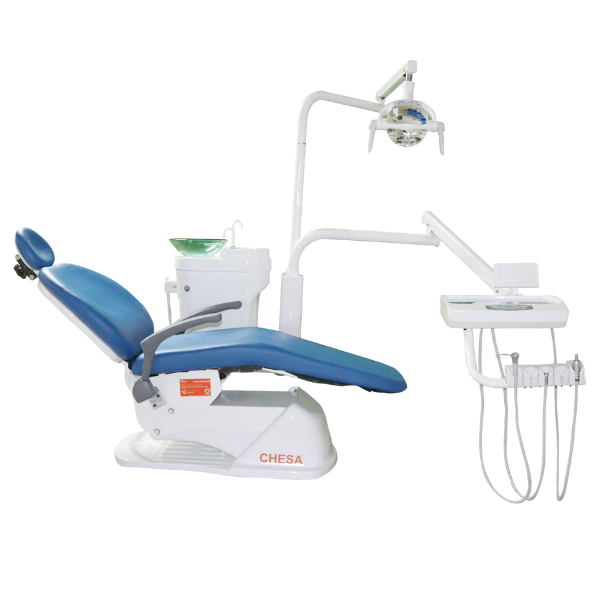
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – CHESA Dental Chair Procurement (2026 Edition)
Frequently Asked Questions: CHESA Dental Chair Acquisition in 2026
As a leading provider of advanced dental solutions, CHESA continues to deliver high-performance dental chairs engineered for durability, ergonomics, and clinical efficiency. Below are five critical FAQs for dental clinics and distributors evaluating CHESA dental chairs in 2026, focusing on voltage compatibility, spare parts availability, installation, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements do CHESA dental chairs meet for international installations in 2026? | CHESA dental chairs are engineered to support global deployment with dual-voltage compatibility (110–120V and 220–240V, 50/60 Hz). All units are equipped with automatic voltage detection and include region-specific power adapters to ensure compliance with local electrical standards (e.g., CE, UL, CCC). Voltage specifications are clearly labeled on the device and in the technical datasheet provided with each shipment. |
| 2. Are spare parts for CHESA dental chairs readily available, and what is the lead time for critical components? | Yes. CHESA maintains a global spare parts network with regional distribution hubs in North America, Europe, and Asia. Common components (e.g., handpieces, upholstery kits, control panels) are available within 48–72 hours. Critical mechanical or electronic parts (e.g., hydraulic pumps, circuit boards) are stocked with a standard lead time of 5–7 business days. Distributors receive priority access through the CHESA Partner Portal for real-time inventory tracking and expedited logistics. |
| 3. Does CHESA provide professional installation services, and what does the process entail? | CHESA offers certified installation services through its global network of trained technicians. The installation includes unboxing, mechanical assembly, electrical and water line integration (if applicable), calibration of chair functions, and operator training. For clinics with in-house technical staff, CHESA provides detailed installation manuals and remote support. Installation is recommended within 14 days of delivery to activate full warranty coverage. |
| 4. What is the standard warranty period for CHESA dental chairs in 2026, and what does it cover? | CHESA provides a comprehensive 3-year limited warranty on all dental chairs purchased in 2026. This includes coverage for manufacturing defects in materials and workmanship, including the base, frame, lifting mechanism, electrical systems, and upholstery. The warranty excludes damage from improper use, unauthorized modifications, or lack of maintenance. Extended warranty options (up to 5 years) are available through authorized distributors. |
| 5. How does CHESA support clinics and distributors with post-warranty maintenance and repair services? | CHESA offers a global service support program including post-warranty maintenance contracts (PMCs), on-demand repair services, and discounted spare parts for legacy models. All repairs are performed by CHESA-certified technicians using OEM parts to maintain performance integrity. Distributors can enroll clinics in CHESA Care Plans, which include annual inspections, software updates (for digital models), and priority response times (under 48 hours in most regions). |
Need a Quote for Chesa Dental Chair Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


