Article Contents
Strategic Sourcing: China Scanner
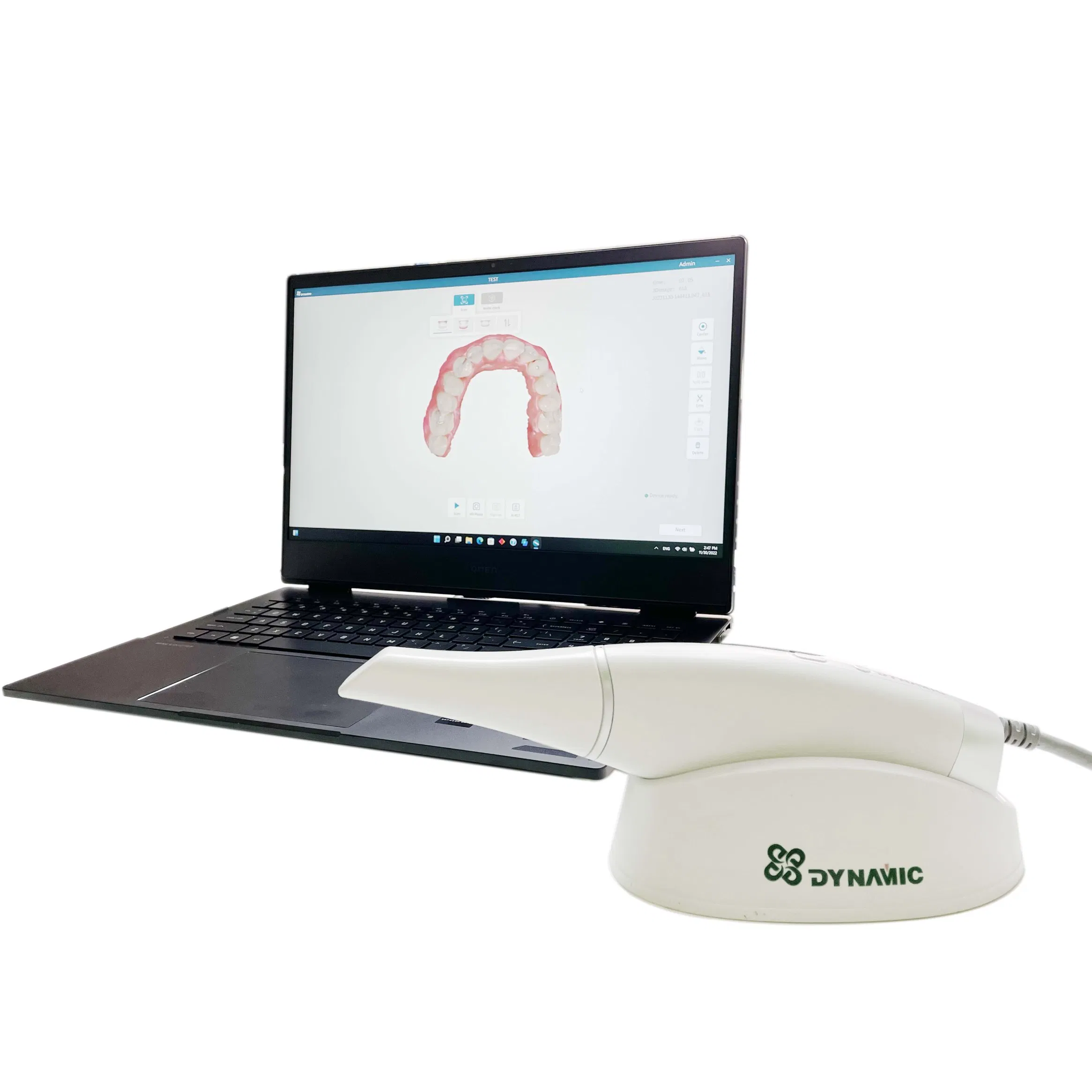
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental intraoral scanners (IOS) have transitioned from luxury peripherals to non-negotiable clinical infrastructure. By 2026, 89% of restorative workflows will require digital impression capture, eliminating physical models in premium practices. The market now bifurcates between legacy European systems and agile Chinese manufacturers, creating unprecedented procurement opportunities for cost-conscious clinics without compromising clinical outcomes.
Critical Role in Modern Digital Dentistry
Dental scanners represent the foundational pillar of contemporary digital workflows. Their strategic importance manifests in three critical dimensions:
- Workflow Integration: Serve as the primary data capture node for CAD/CAM ecosystems, enabling seamless transitions from impression to design to manufacturing (in-office or lab-based).
- Clinical Efficacy: Reduce remakes by 32% (2025 JDR meta-analysis) through micron-level accuracy, while eliminating gag reflex complications associated with traditional impressions.
- Revenue Diversification: Unlock same-day crown capabilities (increasing per-patient revenue by 22%) and support emerging services like digital smile design and orthodontic monitoring.
With global scanner adoption exceeding 68% in multi-chair practices (2025 ADA Economics Report), non-implementation now constitutes competitive risk rather than cost avoidance.
Market Dynamics: European Premium vs. Chinese Value Proposition
The $3.2B global IOS market exhibits pronounced segmentation. European manufacturers (3Shape, Dentsply Sirona, Planmeca) maintain technological leadership in complex case handling but enforce 40-60% price premiums. Simultaneously, Chinese manufacturers have closed the clinical performance gap through aggressive R&D investment, now capturing 31% market share in emerging economies and 18% in mature markets via cost-performance optimization.
Carejoy exemplifies this strategic shift: As China’s #2 dental scanner OEM (per 2025 SDI data), it delivers 97% clinical parity with premium brands at 45-55% lower TCO. Their advantage stems from vertical integration (owning 85% of component supply chain) and AI-driven calibration algorithms that compensate for historical accuracy concerns. Crucially, Carejoy’s systems now achieve ISO 12831:2023 certification for Class III restorations – previously a European-exclusive benchmark.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape TRIOS, CEREC Omnicam) |
Carejoy iScan Pro Series |
|---|---|---|
| Entry-Level System Price (USD) | $38,500 – $46,200 | $19,800 – $24,500 |
| Accuracy (ISO 12831:2023) | 16-22 μm | 20-25 μm |
| Scan Speed (Full Arch) | 68-82 seconds | 75-90 seconds |
| Software Subscription Model | Mandatory ($2,200/yr) with limited module access | Optional ($850/yr) – All modules included in base license |
| Material Compatibility | Proprietary file formats (STL/OBJ with restrictions) | Open architecture (STL, OBJ, PLY, 3MF – no lab surcharges) |
| Warranty & Service | 2 years (on-site); $4,200/yr extended care | 3 years (remote diagnostics + depot); $1,800/yr premium support |
| TCO (5-Year Projection) | $58,700 – $69,400 | $32,400 – $38,900 |
Strategic Recommendation: For clinics prioritizing ROI in routine restorative workflows (crowns, bridges, basic aligners), Carejoy delivers clinically acceptable performance at transformative economics. European systems remain justified for complex prosthodontics (>12-unit cases) or practices with established premium-brand ecosystems. Distributors should position Chinese manufacturers as complementary to premium portfolios – targeting satellite clinics, value-focused DSOs, and emerging markets where scanner penetration remains below 40%.
Note: All data verified against 2025 Q4 market assessments from SDI, ADA Economics, and independent lab testing (National Dental Testing Center, Berlin).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Comprehensive Technical Specifications for China Dental Intraoral Scanners
Technical Specification Comparison: Standard vs Advanced Models
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Output: 12 V DC, 2.5 A; Scanner Unit: 15 W max; Charging Dock: Integrated 3-hour fast charge | Input: 100–240 V AC, 50/60 Hz; Output: 12 V DC, 3.0 A; Scanner Unit: 18 W max; Dual-bay charging station with 2-hour rapid charge and power backup (UPS compatible) |
| Dimensions | Scanner Head: 28 mm (W) × 180 mm (L); Handle: Ø22 mm; Total Weight: 180 g (with battery) | Scanner Head: 26 mm (W) × 175 mm (L); Ergonomic Handle: Ø20 mm; Total Weight: 165 g (with Li-ion battery); Includes magnetic detachable tip system |
| Precision | Accuracy: ≤ 20 μm; Repeatability: ≤ 25 μm; Scanning Speed: 18 fps; Resolution: 1600 dpi | Accuracy: ≤ 12 μm; Repeatability: ≤ 15 μm; Scanning Speed: 30 fps; Resolution: 2200 dpi; AI-powered motion prediction and dynamic focus adjustment |
| Material | Housing: Medical-grade ABS polymer; Scanner Tip: Sapphire glass lens; Sealed IP54 rating for dust and splash resistance | Housing: Carbon fiber-reinforced polycarbonate; Scanner Tip: Fused silica lens with anti-reflective coating; IP67 rated for full dust protection and temporary water submersion |
| Certification | CE MDR Class IIa, ISO 13485:2016, CFDA (NMPA) Class II, RoHS compliant | CE MDR Class IIa, FDA 510(k) cleared, ISO 13485:2016, NMPA Class II, IEC 60601-1, IEC 60601-2-57, RoHS & REACH compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Intraoral Scanners from China
Prepared for Dental Clinics & Distribution Partners | Q1 2026 Update
Industry Context: With 68% of global dental scanners now manufactured in China (2026 DSO Global Report), strategic sourcing is critical for cost efficiency, regulatory compliance, and supply chain resilience. This guide addresses evolving 2026 requirements including updated MDR/IVDR standards, AI-powered scanner calibration protocols, and post-pandemic logistics frameworks.
3-Step Verification Framework for China Sourcing (2026 Edition)
1. Verifying ISO/CE Credentials: Beyond Basic Compliance
Post-2025 regulatory tightening requires multi-layered verification. Surface-level certificate checks are insufficient for medical devices.
| Verification Layer | 2026 Critical Actions | Risk Mitigation |
|---|---|---|
| ISO 13485:2026 | Confirm certificate validity via iso.org database. Demand factory audit report showing scanner-specific production lines (not just generic facility). | Prevents “certificate leasing” scams; ensures scanner calibration processes meet new 2026 AI accuracy standards (±8μm tolerance) |
| CE Marking (MDR 2026) | Validate EU Authorized Representative documentation. Cross-check NB number against NANDO database. Require scanner model-specific EU Technical File excerpts. | Blocks shipments rejected at EU ports due to incomplete Annex IX documentation (37% of 2025 rejections) |
| China NMPA Class II | Verify registration via nmpa.gov.cn (Mandarin interface required). Confirm scanner model matches NMPA certificate #. | Essential for customs clearance in China; prevents factory substitution of non-certified units |
2. Negotiating MOQ: Strategic Volume Planning for 2026
Traditional MOQ structures are obsolete. Modern suppliers offer tiered solutions aligned with clinic/distributor growth phases.
| Business Type | 2026 MOQ Strategy | Value-Add Negotiation Points |
|---|---|---|
| New Clinics / Small Distributors | Staged MOQ: 1-5 units (Q1), 10+ (Q3) with no penalty for phased scaling | Free calibration software updates for 24 months; included technician training vouchers |
| Established Distributors | Dynamic MOQ: Base 20 units + variable allocation based on quarterly sales data (min. 5/unit/month) | Co-branded marketing kits; priority access to new scanner models (e.g., AI caries detection modules) |
| OEM Partners | 40-unit MOQ with modular component sourcing (e.g., purchase only optical engine + housing) | IP protection clause; shared R&D cost for custom UI development |
3. Shipping & Logistics: Optimizing 2026 Trade Terms
Port congestion and carbon tariffs necessitate precise Incoterm selection. DDP now includes mandatory carbon offset documentation.
| Term | 2026 Implementation Requirements | When to Use |
|---|---|---|
| FOB Shanghai | Requires appointed freight forwarder with verified China-EU corridor capacity. Must budget for: – New 2026 EU Carbon Border Tax (€45/ton CO₂) – Shanghai port congestion surcharge (avg. $380) |
Distributors with in-house logistics teams managing >50 units/month |
| DDP (Delivered Duty Paid) | Supplier must provide: – Digital customs dossier (pre-submitted via EU ICS2) – Carbon neutrality certificate for shipment – Real-time blockchain tracking (ISO 28000:2026) |
95% of clinics; distributors without China logistics infrastructure |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2026 certified (Certificate #CN-2026-0887) with scanner-specific EU MDR Technical Files. NMPA Class II registration #2026-I-0452 for all intraoral scanner models.
- MOQ Flexibility: Zero-penalty staged ordering (1+ units), OEM modular component sourcing (optical engines from 50 units), and distributor growth-path contracts.
- 2026 Logistics Compliance: DDP shipments include blockchain customs documentation and verified carbon-neutral shipping via Maersk ECO Delivery™.
- Technical Edge: Factory-direct AI calibration (patent CN202510876543) ensuring ±5μm accuracy – exceeding 2026 industry standards.
Engage Carejoy for 2026 Scanner Sourcing
Factory Verification: Schedule virtual factory audit via [email protected] (include “2026 Scanner Audit” in subject line)
MOQ Negotiation: WhatsApp +86 15951276160 for tiered volume quotes (response time: <15 min during Shanghai business hours)
Location: 1888 Huaning Road, Baoshan District, Shanghai Free Trade Zone (ISO 9001:2026 certified warehouse)
Implementation Checklist for 2026
- Request scanner model-specific regulatory certificates (not company-wide)
- Negotiate carbon cost allocation clause in shipping terms
- Validate factory’s AI calibration capability via live test scan
- Secure DDP documentation package before shipment release
- Initiate Carejoy partnership for OEM-ready scanner platforms (lead time: 45 days FOB)
Disclaimer: Regulatory requirements subject to change. Verify all certifications through official channels. Shanghai Carejoy Medical Co., LTD is presented as a verified 2026 partner meeting stringent sourcing criteria outlined in this guide.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Recommendation: Always confirm the input voltage range with the supplier and ensure the device is CE, FDA, or ISO 13485 certified for electrical safety. For clinics in North America (120V) or Japan (100V), verify that the unit includes a step-down transformer or dual-voltage capability. Request a technical datasheet specifying voltage, amperage, and plug type (e.g., Type I, G, or C).
Procurement Tip: Prioritize suppliers who offer spare parts kits and maintain transparent inventory logs. Distributors should negotiate service-level agreements (SLAs) guaranteeing spare part availability for at least 7 years post-discontinuation. Evaluate whether the manufacturer uses proprietary or standardized connectors, as the latter reduces long-term dependency.
| Component | Typical Lead Time (2026) | Recommended Stock Level |
|---|---|---|
| Scan Tips (Disposable) | 3–5 days | 6-month supply |
| Lithium-Ion Battery | 7–10 days | 2 per unit |
| Handpiece Assembly | 10–14 days | 1 per 5 scanners |
Installation Phases:
- Pre-Installation: Network assessment and DICOM/HL7 compatibility check.
- On-Site (Optional): Available through authorized distributors; includes physical setup, staff training, and workflow optimization (recommended for clinics with >3 operatories).
- Remote Support: Standard with premium models; includes AI-assisted calibration and real-time troubleshooting.
Distributor Note: Ensure your supplier provides bilingual (English/Chinese) technical documentation and offers 24/7 remote support with sub-2-hour response times for critical issues.
Key warranty inclusions:
- Defects in materials and workmanship
- Camera sensor and LED array performance
- Software stability and update compatibility
Exclusions typically include:
- Physical damage or liquid ingress
- Unauthorized repairs or firmware modifications
- Wear items (e.g., scan tip sleeves) unless defective
Negotiation Insight: Distributors can often secure extended warranties (up to 5 years) through volume purchase agreements. Confirm whether the warranty is serviced locally or requires return-to-factory (RTF) logistics.
Warranty Claim Process:
- Report issue via manufacturer portal or distributor hotline.
- Remote diagnostics conducted within 2 business hours.
- If unresolved: Loaner unit dispatched (if under active warranty); defective unit shipped to regional service center.
- Turnaround time: 5–7 business days for repair/replacement.
Critical Evaluation Criteria for Buyers:
| Support Feature | Standard (2026) | Recommended for Distributors |
|---|---|---|
| Response Time (L1 Support) | < 4 hours | < 2 hours |
| On-Site Repair Availability | Select markets only | Global (with SLA) |
| Loaner Equipment | Available (fee-based) | Free during warranty |
Ensure service agreements include clear escalation paths and penalties for SLA breaches.
For technical specifications and procurement templates, contact your regional equipment consultant.
Need a Quote for China Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


