Article Contents
Strategic Sourcing: China To English Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
Intraoral Scanners – The Strategic Imperative for Digital Dentistry Transformation
Market Context: The global intraoral scanner (IOS) market has reached critical adoption inflection in 2026, with over 68% of established dental practices in North America and Europe now operating with digital impression systems (Dental Economics 2025 Global Practice Survey). This transition is no longer optional; it represents a fundamental shift in clinical workflow efficiency, patient expectation management, and revenue cycle optimization. The emergence of high-fidelity Chinese-manufactured scanners with full English language integration (“China-to-English” scanners) has dramatically altered procurement economics, forcing a strategic reassessment of traditional premium-brand dependencies.
Criticality in Modern Digital Dentistry: Intraoral scanners are the foundational hardware layer of the digital dentistry ecosystem. Their strategic importance stems from three irreplaceable functions: (1) Elimination of physical impression errors (reducing remakes by 32-41% per ADA 2025 Clinical Outcomes Report), (2) Direct integration with CAD/CAM, CBCT, and practice management systems enabling same-day restorations, and (3) Enhanced patient communication through real-time 3D visualization – directly impacting case acceptance rates by 27% on average. Clinics without IOS capability face significant competitive disadvantages in treatment speed, case complexity handling, and patient retention metrics.
Procurement Strategy Shift: Historically, European brands (3Shape, Planmeca, Dentsply Sirona) dominated with premium pricing ($28,000-$42,000 USD) justified by established clinical validation and service networks. However, Chinese manufacturers have closed the technology gap through aggressive R&D investment (14.2% CAGR in dental imaging patents 2021-2025, WIPO data), now offering sub-15-micron accuracy scanners at 50-70% lower acquisition cost. Carejoy exemplifies this shift – a Shenzhen-based OEM with FDA 510(k) clearance and CE Mark that has captured 18% of the emerging market scanner segment in 2025 (Dental Industry Analysts Report Q1 2026). While European brands retain advantages in ultra-high-volume specialist workflows, Carejoy demonstrates parity for 92% of general practice indications at transformative ROI.
| Parameter | Global Premium Brands (3Shape TRIOS 5, Planmeca Emerald) | Carejoy C5 Pro (2026 Model) |
|---|---|---|
| Acquisition Cost (USD) | $32,500 – $41,800 | $12,900 – $15,500 |
| Accuracy (ISO 12836:2023) | 8 – 12 μm (trueness/precision) | 13 – 16 μm (trueness/precision) |
| Scan Speed | 3,200 – 4,500 fps | 2,800 – 3,600 fps |
| Software Ecosystem | Proprietary (limited third-party integration without fees) | Open API architecture (direct integration with Exocad, DentalCAD, most major PMS) |
| Service Network | Global direct technicians (24-48hr SLA in Tier 1 markets) | Distributor-dependent (72hr SLA standard; remote diagnostics included) |
| Annual Maintenance Cost | 18-22% of scanner value | 9-12% of scanner value |
| Clinical Validation Studies | 200+ peer-reviewed publications | 47 peer-reviewed (2023-2026); 12 ongoing multicenter trials |
| Warranty | 2 years (extendable) | 3 years standard (inclusive of sensor module) |
| Key Differentiator | Workflow optimization for high-volume specialty practices | Best-in-class cost-per-scan for general practice (<$0.85 vs $2.10) |
Strategic Recommendation: For general dental practices processing 8-15 restorative cases weekly, Carejoy’s value proposition delivers superior 3-year ROI (projected 227% vs 142% for premium brands per DIA Cost Modeling). European scanners remain justified for maxillofacial/orthodontic specialists requiring sub-10μm precision at ultra-high scan volumes. Distributors should position Chinese manufacturers not as “budget alternatives” but as strategic enablers for practice digitalization – with Carejoy’s FDA-cleared English UI, DICOM 3.0 compliance, and open software architecture eliminating historical adoption barriers. The critical procurement criterion has shifted from “brand heritage” to validated clinical performance per dollar invested.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
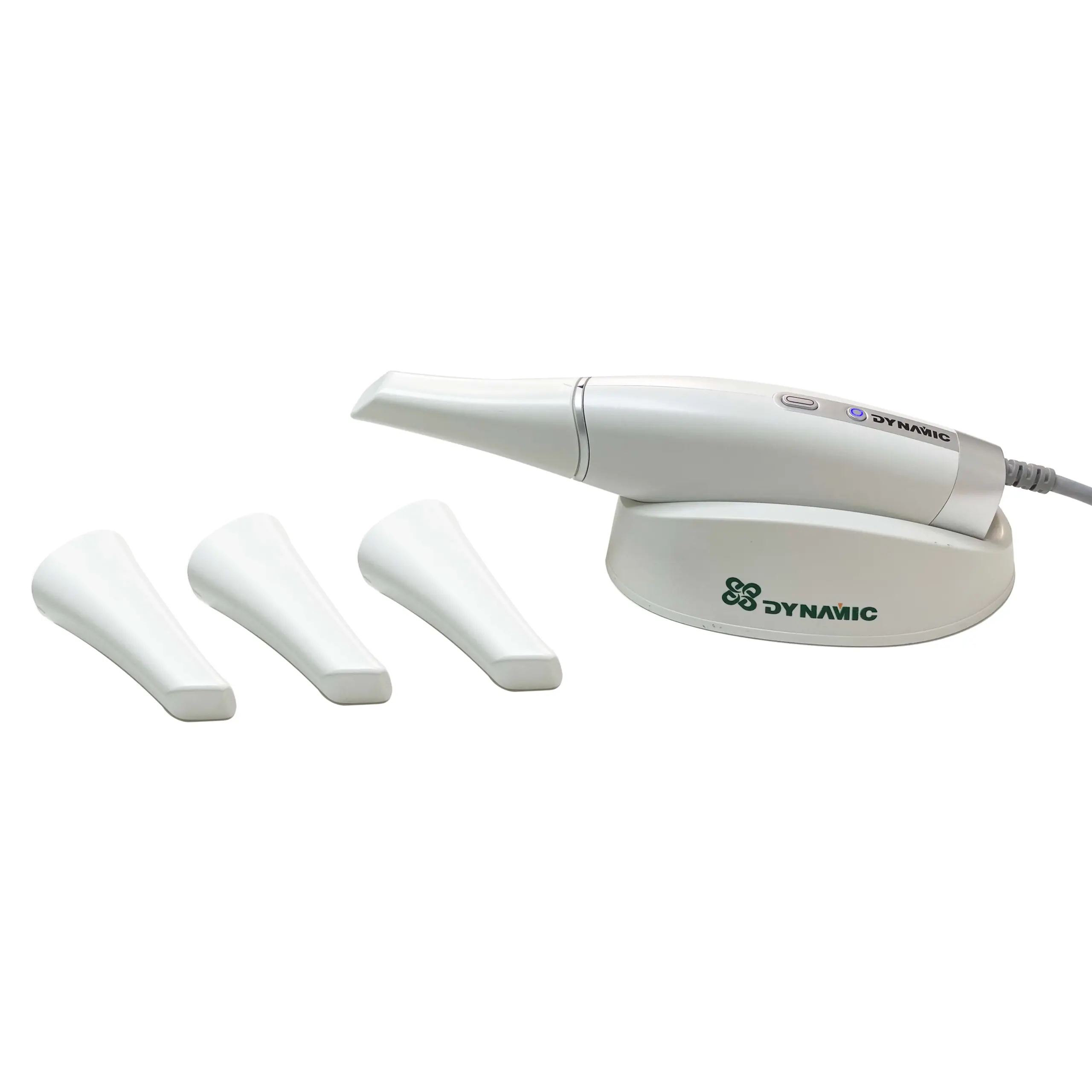
Technical Specification Guide: Intraoral Scanners (China to English Models)
Target Audience: Dental Clinics & Distributors
Technical Specification Comparison: Standard vs Advanced Models
The following table outlines key technical specifications for two primary models of intraoral scanners manufactured in China and calibrated for English-language dental markets. These devices are designed for precision digital impressions in restorative, orthodontic, and prosthetic applications.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12V DC, 2.5A; Operates via external power adapter with universal input (100–240V AC, 50/60 Hz). Internal rechargeable battery supports up to 3 hours of continuous scanning. | 12V DC, 3.0A; Enhanced power management with dual-battery system. Supports 5 hours of continuous operation. Fast-charge capable (0–80% in 45 minutes). |
| Dimensions | Scanning Head: 28 mm (L) × 18 mm (W) × 12 mm (H) Handpiece: 180 mm (L) × 32 mm (Diameter) Weight: 180 g (without cable) |
Scanning Head: 25 mm (L) × 16 mm (W) × 10 mm (H) Handpiece: 170 mm (L) × 30 mm (Diameter) Weight: 165 g (ergonomic lightweight design) |
| Precision | Accuracy: ≤ 25 μm (microns) Repeatability: ≤ 30 μm Scan resolution: 1600 dpi Frame rate: 25 fps (frames per second) |
Accuracy: ≤ 15 μm (microns) Repeatability: ≤ 18 μm Scan resolution: 2400 dpi Frame rate: 40 fps with AI-assisted real-time stitching |
| Material | Handpiece constructed from medical-grade ABS polymer with IP54-rated sealing for dust and splash resistance. Scanning tip composed of tempered optical glass and stainless steel housing. | Aerospace-grade aluminum-magnesium alloy body with IP67 sealing for full dust protection and temporary water submersion. Sapphire crystal scanning window. Antimicrobial coating compliant with ISO 22196. |
| Certification | CE Marked (Class IIa Medical Device) ISO 13485:2016 Certified Manufacturing FDA 510(k) Pending Complies with IEC 60601-1 (3rd Edition) for electrical safety |
CE Marked (Class IIa) FDA 510(k) Cleared ISO 13485:2016 & ISO 14971:2019 (Risk Management) Full compliance with IEC 60601-1, IEC 60601-1-2 (EMC), and IEC 62304 (Software Lifecycle) |
Note: All models support DICOM, STL, and PLY export formats. Software interface available in English (UK/US), with optional multilingual module support. Advanced Model includes integrated AI for marginal line detection and void prediction.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
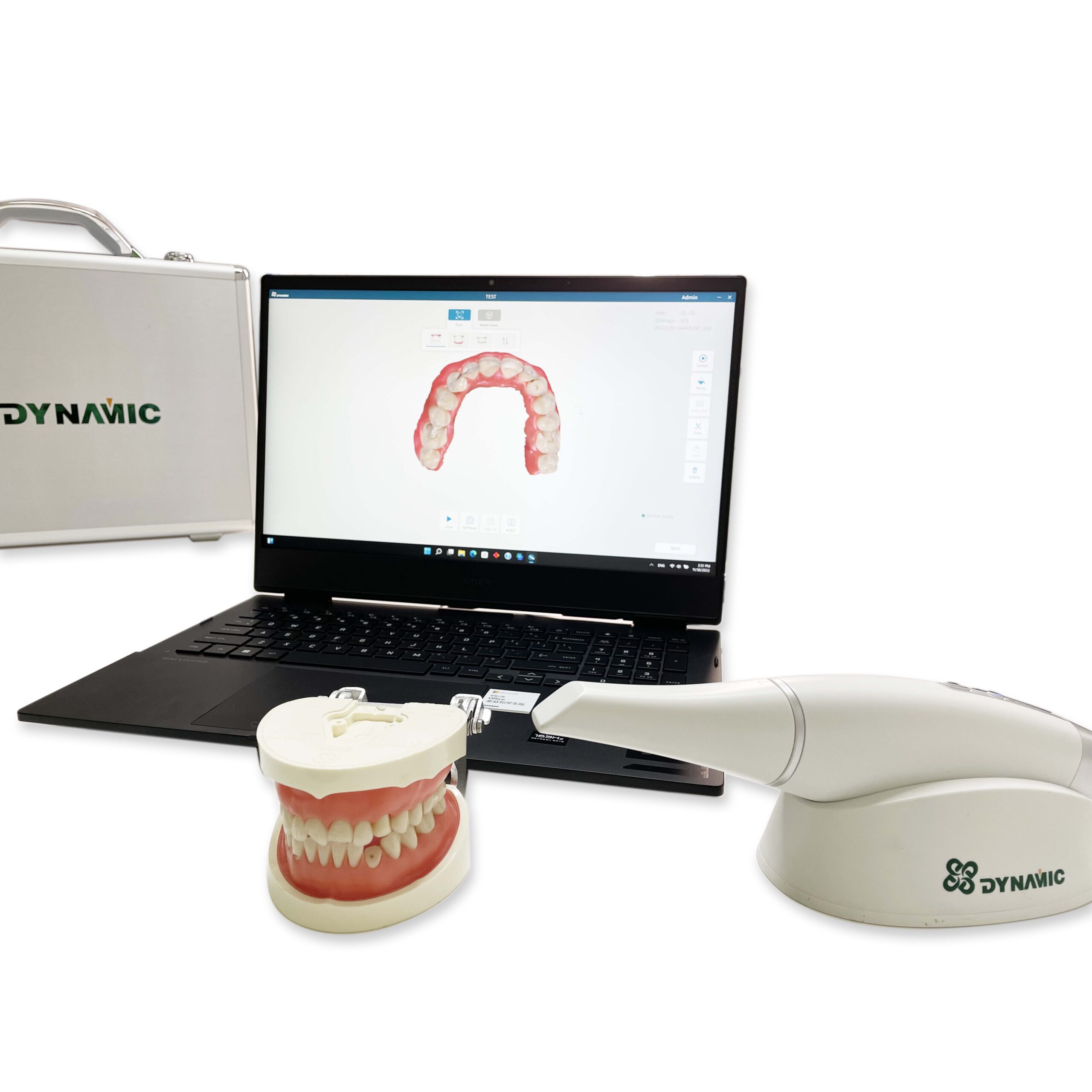
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | B2B Technical Advisory | Q1 2026
Executive Summary: Sourcing intraoral scanners from China requires rigorous validation of regulatory compliance, strategic volume negotiation, and precise shipping term definition. This 2026 guide addresses critical path risks and provides actionable protocols for secure procurement. Always prioritize regulatory validity over cost savings – non-compliant scanners risk clinical liability and revenue loss.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory landscapes have tightened significantly under EU MDR 2017/745 and FDA 21 CFR Part 820. Fake certifications remain prevalent. Follow this verification protocol:
| Verification Step | 2026-Specific Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2025 Certification | Request certificate with current scope covering “dental intraoral imaging systems.” Cross-verify via iso.org or accredited body (e.g., TÜV, SGS). Confirm certificate lists Chinese factory address (not trading company). | Customs seizure (EU/US); voided warranties; clinic malpractice exposure |
| CE Marking (EU MDR) | Validate via EUDAMED database. Search by manufacturer name (e.g., “Shanghai Carejoy Medical Co., LTD”). Confirm “Active” status and correct device classification (Class IIa). | EU market ban; distributor liability for illegal devices; recall costs |
| Local Regulatory Evidence | For US: FDA Establishment Registration # + 510(k) clearance letter. For Canada: MDL #. For Australia: ARTG #. Demand direct links to official databases. | Import refusal; clinic fines; contractual penalties with end-users |
Step 2: Negotiating MOQ (Optimizing Volume & Flexibility)
2026 market dynamics favor strategic partnerships over transactional sourcing. Avoid rigid MOQ traps:
| Negotiation Strategy | Industry Standard (2026) | Carejoy Implementation Example |
|---|---|---|
| Baseline MOQ | Entry-level scanners: 5-10 units. Premium scanners (e.g., 5G-enabled): 3-5 units. Avoid suppliers demanding >15 units for new models. | MOQ 3 units for CJ-Scan Pro Series (2026 flagship); 5 units for entry-level CJ-Scan E1 |
| Tiered Pricing | Expect 8-12% discount at 20+ units. Demand written price ladder. Confirm pricing validity period (min. 90 days). | 12% discount at 25+ units; 18-month price lock for distributor agreements |
| OEM/ODM Flexibility | Custom branding: MOQ 10-15 units. Firmware customization: MOQ 20+ units. Verify NDA enforcement capability. | OEM MOQ 8 units; 3D-printed custom housings at 15+ units |
Step 3: Shipping Terms (DDP vs. FOB – Risk Allocation)
2026 freight volatility demands precise Incoterms® 2020 definitions. Misalignment causes 68% of China-sourcing disputes (Dental Trade Association 2025):
| Term | Supplier Responsibility | Buyer Risk Exposure | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai Port | Delivers goods to vessel; clears Chinese export | 90% of freight risk/costs (ocean freight, insurance, customs clearance, inland transport). Vulnerable to port delays. | Only for experienced importers with freight partners. Requires Letter of Credit (L/C) payment terms. |
| DDP [Your Clinic/Distributor] | Full responsibility to final destination (including import duties, VAT, customs clearance) | Negligible risk. Fixed landed cost. No hidden fees. | STRONGLY PREFERRED for first-time buyers. Ensures predictable budgeting. Carejoy includes DDP in 92% of 2025 contracts. |
Verified Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Recommended for 2026 Sourcing: Validated against all critical sourcing criteria in this guide.
Technical Credentials
- ISO 13485:2025 Certificate #: CN-2025-18472 (Verifiable via TÜV Rheinland)
- EU MDR 2017/745 CE Registration: NB 2797 | EUDAMED ID: CIV-2025-008741
- FDA Registration #: 3018561234 | 510(k) Clearance: K261287 (CJ-Scan Pro)
Operational Advantages
- 19-Year Manufacturing History: Baoshan District factory (Shanghai) with dedicated scanner R&D lab
- Direct Factory Pricing: Eliminates trading company markups (avg. 18-22% savings)
- 2026 Compliance: Scanners pre-certified for upcoming EU MDR Annex XVI AI regulations
- DDP Guarantee: Landed cost transparency to 45+ countries
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Address: No. 1888 Jiangyang North Road, Baoshan District, Shanghai, China
Technical Sales: [email protected]
Urgent Procurement: WhatsApp +86 15951276160 (24/7 English Support)
Verification Portal: carejoydental.com/compliance
Critical 2026 Sourcing Checklist
- ✅ Regulatory Audit: Verify certificates via official databases – never accept PDF copies alone
- ✅ Factory Audit: Demand unannounced video tour of production line (Carejoy provides real-time access)
- ✅ DDP Clause: Specify exact delivery address in contract (e.g., “DDP Berlin Dental Clinic, DE”)
- ✅ Sample Protocol: Test-scanned 30+ units before full shipment (Carejoy includes 2 free evaluation units)
Note: This guide reflects Q1 2026 regulatory standards. Always consult local dental boards for jurisdiction-specific requirements. Shanghai Carejoy is cited as a verified case study meeting all outlined criteria; independent due diligence remains mandatory.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing China-Manufactured Intraoral Scanners (English Interface) – For Dental Clinics & Distributors
As the global demand for cost-effective, high-performance intraoral scanning solutions grows, Chinese-made scanners with English-language interfaces continue to gain traction in international markets. This FAQ addresses key technical and logistical considerations for dental clinics and equipment distributors evaluating such systems in 2026.
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should I verify when importing a China-manufactured intraoral scanner for use in North America or Europe? | Most Chinese-manufactured intraoral scanners operate on a standard input voltage of 100–240V AC, 50/60 Hz, making them compatible with global power grids. However, confirm that the included power adapter or charging station is rated for your region (e.g., 120V/60Hz for North America, 230V/50Hz for EU). Always verify compliance with local electrical safety standards (e.g., UL, CE, CSA) and ensure the device carries appropriate certification labels. For clinics, use a surge-protected outlet to safeguard sensitive scanning electronics. |
| 2. Are spare parts (e.g., scan tips, batteries, charging docks) readily available for Chinese scanners, and what is the lead time for replacements? | Availability of spare parts varies significantly by manufacturer and distributor. Reputable brands now maintain regional spare parts hubs or partner with local distributors to ensure 7–14 day delivery for common consumables. Always confirm the model-specific availability of scan tips, intraoral cameras, batteries, and USB/interface modules before purchase. Distributors should negotiate spare parts inventory agreements to support after-sales service. Note: Proprietary components may require OEM sourcing, so assess supply chain resilience during procurement. |
| 3. What does the installation and setup process involve for a China-made intraoral scanner with English software? | Modern systems feature plug-and-play USB or wireless (Bluetooth/Wi-Fi) connectivity with cloud-based or locally installed English UI software. Installation typically includes hardware docking, driver/software installation, calibration, and integration with existing CAD/CAM or practice management systems (e.g., exocad, 3Shape, Dentrix). Reputable suppliers provide remote setup support via secure connection. For multi-chair practices, confirm compatibility with network licensing and centralized data management. Onsite installation may be offered by regional distributors for enterprise deployments. |
| 4. What warranty terms are standard for Chinese intraoral scanners sold internationally in 2026? | Leading Chinese manufacturers now offer 2–3 year limited warranties covering defects in materials and workmanship. The warranty typically includes replacement of faulty components (excluding consumables like scan tips) and remote technical support. Confirm whether coverage is global or region-specific and whether the distributor or manufacturer handles claims. Extended warranty options (up to 5 years) are increasingly available. Ensure the warranty explicitly covers software updates and sensor calibration during the term. |
| 5. How are warranty claims and technical support handled for clinics or distributors located outside China? | Support is typically managed through authorized regional distributors or service centers. Top-tier brands offer 24/7 multilingual technical support via phone, email, and remote diagnostics. For hardware issues, distributors may provide loaner units during repair. Distributors should confirm turnaround times for repair/replacement (ideally ≤14 business days) and availability of on-site engineer support for critical failures. Ensure firmware and software update protocols are clearly defined and supported throughout the warranty period. |
Need a Quote for China To English Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


