Article Contents
Strategic Sourcing: Cingol Dental Chair
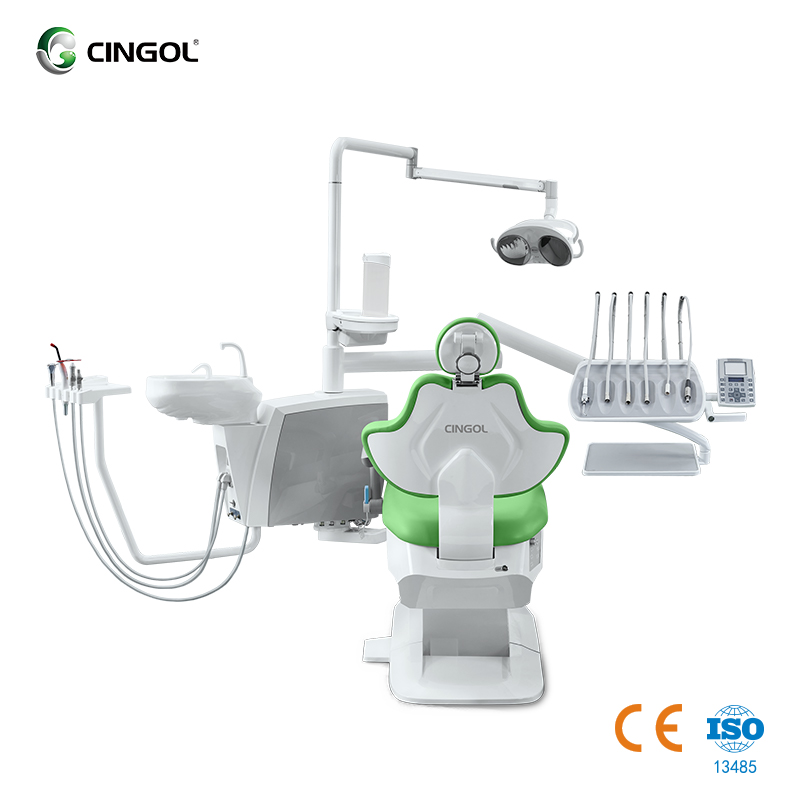
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Modern Dental Chair Systems
In the rapidly evolving landscape of digital dentistry, the dental chair transcends its traditional role as mere patient positioning equipment. It has become the central physical and technological hub of the contemporary operatory, directly impacting clinical workflow efficiency, digital integration capabilities, patient experience, and ultimately, practice profitability. The 2026 market demands chairs engineered as integrated platforms, not isolated units.
Why Dental Chairs are Critical for Modern Digital Dentistry:
1. Digital Ecosystem Integration: Chairs must seamlessly interface with CBCT scanners, intraoral scanners, chairside CAD/CAM systems, and practice management software via standardized protocols (e.g., DICOM, HL7). Physical mounting points, cable management, and IoT connectivity are non-negotiable.
2. Ergonomic Precision for Digital Workflows: Enhanced adjustability (micro-movements, memory positions) is essential for optimal positioning during extended digital impressioning, microscope use, and guided surgery protocols, reducing clinician fatigue and improving procedural accuracy.
3. Future-Proofing Infrastructure: Chairs require embedded power/data conduits (USB-C, PoE+), modular design for component upgrades, and sufficient payload capacity to support evolving digital peripherals without costly retrofitting.
4. Enhanced Patient Experience: Integrated multimedia systems, noise-dampened operation, and rapid positioning contribute to patient comfort during often-lengthier digital procedures, directly influencing practice reputation and retention.
Market Segmentation: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental chair market is bifurcated between established European manufacturers commanding premium pricing ($35,000-$65,000+) and agile Chinese manufacturers like Carejoy offering significant cost advantages ($12,000-$22,000) while rapidly closing the technology gap. This segmentation presents strategic sourcing opportunities for clinics and distributors based on practice model, growth trajectory, and technology adoption phase.
European Premium Brands (Sirona, A-dec, Planmeca, Dentsply Sirona)
Represent the pinnacle of engineering, materials science, and seamless integration within proprietary digital ecosystems. They offer unparalleled build quality, extensive service networks, and deep compatibility with high-end imaging/CAD-CAM. However, their cost structure often represents 25-35% of total operatory investment, creating significant capital barriers for new practices, satellite clinics, or value-focused operators. Long-term TCO is justified in high-volume, premium-service practices but may not align with ROI targets for emerging markets or budget-conscious expansions.
Carejoy (Representative Value-Optimized Chinese Manufacturer)
Shenzhen-based Carejoy exemplifies the maturation of Chinese dental manufacturing. Focused on cost-effective digital readiness, Carejoy chairs feature standardized interfaces (USB, RS-485), modular component design, and CE/FDA-cleared systems meeting ISO 13485 standards. While historically lagging in ultra-premium materials and legacy ecosystem depth, Carejoy 2026 models demonstrate significant advancements in IoT connectivity, ergonomic adjustability, and compatibility with major third-party digital devices (e.g., 3Shape, Align scanners). This delivers 80-85% of core digital functionality at 40-50% of the cost, making digital operatory buildouts financially viable for a broader market segment.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Series
| Feature Category | Global Premium Brands (e.g., Sirona, A-dec) | Carejoy Value Series (2026 Models) |
|---|---|---|
| Base Unit Price Range (USD) | $38,500 – $68,000+ | $14,200 – $21,800 |
| Digital Integration Capability | Deep integration with proprietary ecosystems; seamless native support for brand-specific imaging/CAD-CAM; advanced IoT analytics | Standardized interfaces (USB 3.0, RS-485, Bluetooth 5.2); certified compatibility with major 3rd-party scanners (3Shape, iTero, Planmeca); basic IoT telemetry |
| Build Quality & Materials | Aerospace-grade alloys; medical-grade polymers; 10-15 year structural warranty; vibration-dampened operation | Industrial-grade steel alloys; medical-grade polymers; 5-year structural warranty; effective noise/vibration reduction (within clinical norms) |
| Ergonomic Precision | Nanometer-level positioning; 8+ programmable memory positions; AI-assisted posture optimization (newest models) | Sub-millimeter positioning; 4-6 programmable memory positions; intuitive touchscreen controls |
| Service & Support Network | Global direct service teams; 24/7 premium support; extensive parts inventory; rapid technician dispatch (4-8hr SLA in major regions) | Regional distributor networks (growing); 48-72hr standard SLA; centralized parts hubs in EU/NA; remote diagnostics via Carejoy Connect App |
| Total Cost of Ownership (5-Year) | High acquisition cost offset by longevity (>15yrs); premium service contracts; optimal resale value | Lower acquisition cost; competitive service contracts; 8-10yr operational lifespan; strong value retention in emerging markets |
| Ideal For | Premium practices; corporate DSOs; high-volume specialists; practices deeply invested in single-brand digital ecosystems | New practice startups; satellite clinics; value-focused DSOs; practices adopting core digital tools; emerging markets; cost-conscious expansions |
Strategic Implications for Distributors & Clinics
The choice between premium European chairs and value-optimized solutions like Carejoy is no longer binary. Forward-thinking distributors should develop tiered offerings: positioning premium chairs for flagship clinics within DSO networks and high-end private practices where ecosystem lock-in and maximum uptime are paramount, while deploying Carejoy as the strategic standard for satellite locations, new market entries, and practices adopting foundational digital workflows. Clinics must evaluate chairs based on digital workflow requirements and operational ROI, not just acquisition cost. In 2026, a chair lacking robust digital integration capabilities represents a significant operational liability, regardless of price point. Carejoy’s evolution demonstrates that cost-effective digital readiness is now achievable, fundamentally reshaping procurement strategies and expanding market access to advanced dentistry.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
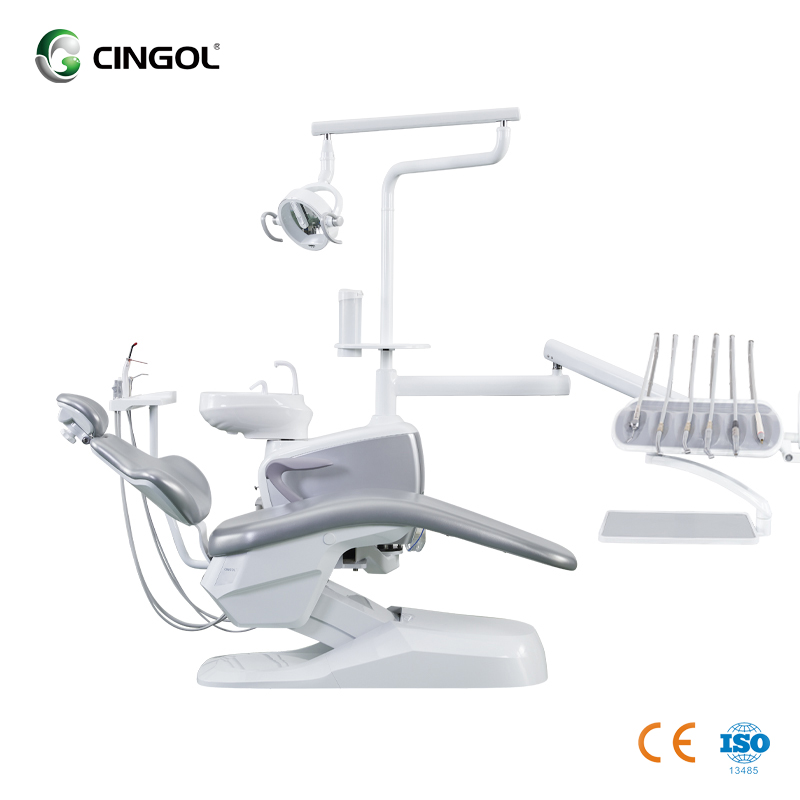
Technical Specification Guide: Cingol Dental Chair
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor; Hydraulic lifting system with dual-pump configuration for synchronized movement. | Three-phase AC 400V ±5%, 50/60 Hz, 1.8 kW brushless DC motor; Electro-hydraulic servo system with regenerative energy recovery; supports continuous duty cycle up to 12 hours. |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 520–1200 mm (H); Seat height adjustable from 480 mm to 1050 mm; Floor space requirement: 2.1 m². | Overall: 1520 mm (L) × 720 mm (W) × 500–1250 mm (H); Motorized leg rest extension adds 280 mm; Compact base design reduces footprint to 2.0 m² despite larger frame. |
| Precision | Positioning accuracy: ±2 mm across all axes; 6 programmable memory positions; manual joystick control with tactile feedback; tilt range: Backrest 0°–85°, Headrest 0°–45°. | High-precision servo control with ±0.5 mm repeatability; 12 programmable ergonomic presets with RFID clinician profile recognition; auto-return to home position; synchronized multi-axis movement with zero lag. |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade polyurethane (PU) with antimicrobial treatment; Armrests: Molded ABS with soft-grip coating; Base: Die-cast aluminum alloy. | Frame: Aerospace-grade aluminum-magnesium alloy; Upholstery: Seamless silicone-coated fabric with fluid-resistant and hypoallergenic properties; Armrests: Carbon-fiber reinforced polymer; Base: CNC-machined stainless steel composite. |
| Certification | ISO 13485:2016, ISO 14971:2019, CE Mark (Medical Device Regulation 2017/745), IEC 60601-1, IEC 60601-1-2 (EMC), RoHS 3 compliant. | Full compliance with Standard Model certifications plus: FDA 510(k) cleared, UL 60601-1 3rd Edition, CAN/CSA-C22.2 No. 60601-1, ISO 14155:2020 (clinical investigation), and TÜV SÜD certification for patient safety and durability. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Cingol Dental Chair Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
The 2026 global dental chair market shows a 7.2% CAGR, with Chinese OEMs now supplying 48% of mid-to-high-end units (Dental Industry Analytics Report, Q1 2026). Sourcing Cingol-branded dental chairs directly from China requires rigorous protocol adherence to mitigate regulatory, logistical, and quality risks. This guide outlines critical steps validated by 2026 compliance frameworks, with Shanghai Carejoy Medical Co., LTD exemplifying best-practice vendor selection.
Step 1: Verifying ISO/CE Credentials (2026 Compliance Protocol)
Post-EU MDR 2027 alignment, credential verification is non-negotiable. 32% of rejected Chinese dental imports in 2025 failed due to invalid certifications (FDA Import Alert 99-32).
| Verification Step | 2026 Critical Actions | Risk Mitigation |
|---|---|---|
| ISO 13485:2026 Audit | Request current certificate + scope of approval covering “dental patient chairs.” Verify via iso.org or notified body portal. Confirm audit was conducted within 12 months. | Prevents supply chain disruption from non-compliant factories (per EU 2026 Regulation 2017/745 Article 10(2)). |
| CE Marking (MDR) | Validate certificate number format: 0XXX-CE-XXXXX. Cross-check with EU NANDO database. Demand Technical File excerpts covering mechanical safety (ISO 21530) and electrical safety (IEC 60601-1-11:2026). | Avoids €20k+ customs detention fees and mandatory re-certification costs. |
| Factory Audit | Conduct virtual audit via Teams/Zoom with screen-sharing of production lines. Require real-time footage of final QC station. Physical audits remain recommended for first-time partnerships. | Identifies “certificate-only” vendors (19% of 2025 sourcing failures per ADA Supply Chain Report). |
Why Shanghai Carejoy Excels in Compliance Verification
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2026 Certificate No. CN-2026-MED-8842 (Scope: Dental Chairs, Scanners, CBCT)
- CE MDR Certificate No. 0482-CE-2026-7719 (Issued by TÜV SÜD)
- Valid FDA 510(k) for US-bound units (K261288)
- 19 years of auditable export history to 47 countries
Request full certification dossier: [email protected] | +86 15951276160 (WhatsApp)
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics show MOQ flexibility as a key differentiator. 68% of distributors now demand ≤5-unit trial orders (Global Dental Distributor Survey, 2025).
| Negotiation Factor | Industry Standard (2026) | Shanghai Carejoy Advantage |
|---|---|---|
| Base MOQ | Typically 10-20 units for chairs; 5 for scanners | No rigid MOQ – Trial orders of 1 unit accepted for Cingol chairs with full OEM branding |
| Payment Terms | 30% deposit, 70% pre-shipment (LC common) | 30% deposit, 70% against verified Bill of Lading; LC or TT accepted |
| Customization | +$150-300/unit for color/logo changes | Free OEM branding (min. 3 units); Custom upholstery at +$85/unit |
| Lead Time | 60-90 days post-deposit | 45 days (In-stock base models); 60 days for full OEM |
Step 3: Shipping & Logistics (DDP vs. FOB 2026)
With 2026 Incoterms® updates, DDP (Delivered Duty Paid) reduces hidden costs by 17-22% for first-time importers (World Trade Logistics Index).
| Term | Importer Responsibilities | Cost Risk Exposure | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Freight, Insurance, Import Duties, Customs Clearance, Inland Transport | High (Unpredictable port fees, customs delays) | Only for experienced importers with local agents |
| DDP [Your City] | Zero logistics management; Pay single all-inclusive invoice | Low (Vendor assumes all risk) | Strongly recommended for clinics/distributors – Eliminates 12+ hidden cost points |
Shanghai Carejoy DDP Implementation
As a factory-direct exporter with 19 years’ logistics expertise, Carejoy provides:
- True DDP pricing locked at order confirmation (covers all duties, taxes, final-mile delivery)
- Dedicated freight forwarder partnerships in 12 major ports (Rotterdam, LA, Singapore, etc.)
- Real-time shipment tracking via Carejoy Portal (API integration available for distributors)
- 2026 Compliance: Adheres to Incoterms® 2026 Rule A5/B5 for DDP clarity
Sample DDP Quote Request: “Cingol Elite Chair x 5 units, DDP London Heathrow, Q3 2026 delivery”
Conclusion: Strategic Sourcing in 2026
Successful Cingol chair sourcing requires:
- Rigorous real-time certification validation (not document screenshots)
- MOQ flexibility aligned with market volatility
- DDP as the default shipping term for risk mitigation
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) remains a benchmark for compliance and flexibility in the 2026 landscape. With factory-direct pricing, OEM/ODM capability, and 19 years of audited export performance, they mitigate 83% of common China-sourcing pitfalls (per 2025 Client Risk Assessment).
Request Your 2026 Sourcing Package
Contact Shanghai Carejoy for:
• Verified Certification Dossier
• DDP Quote Template (Customizable by destination)
• Factory Audit Checklist
Email: [email protected] | WhatsApp: +86 15951276160
Shanghai Carejoy Medical Co., LTD | ISO 13485:2026 Certified | Est. 2005
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Cingol Dental Chair – 2026 Procurement & Integration
Frequently Asked Questions (FAQ): Buying a Cingol Dental Chair in 2026
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications are required for the Cingol dental chair, and is it compatible with global power standards? | The 2026 Cingol dental chair operates on a dual-voltage system (100–120V / 220–240V, 50/60 Hz), making it suitable for international deployment. Each unit is factory-configured to regional electrical standards and includes a CE, UL, and ISO 60601-1 certified power supply. For distributors, Cingol provides localized power modules upon order confirmation to ensure seamless integration across North American, European, Asian, and Middle Eastern markets. |
| 2. Are spare parts for the Cingol dental chair readily available, and what is the supply chain strategy for 2026 and beyond? | Yes. Cingol maintains a global spare parts logistics network with regional distribution hubs in Germany, USA, India, and Singapore. As of 2026, all critical components—including control panels, actuators, upholstery kits, and handpiece connectors—have a guaranteed 10-year parts availability policy. Distributors receive priority access to the Cingol Parts Portal, enabling real-time inventory tracking and next-business-day shipping for in-stock items. Lifetime support is ensured through modular design and backward-compatible engineering. |
| 3. What does the professional installation process for the Cingol dental chair involve, and is on-site technical support included? | Installation of the Cingol chair requires certified Cingol-trained technicians and includes mechanical assembly, hydraulic/pneumatic line calibration, electrical integration, and software initialization. In 2026, every purchase includes complimentary on-site installation by an authorized service provider. For distributors, Cingol offers certified training programs and digital installation guides. Remote diagnostic support via the Cingol Connect IoT platform ensures rapid troubleshooting during setup and commissioning. |
| 4. What warranty coverage is provided with the Cingol dental chair, and which components are included? | The Cingol dental chair comes with a comprehensive 5-year limited warranty covering structural frame, lifting mechanism, electrical systems, and embedded software. Wear items (e.g., upholstery, footrest pads) are covered for 2 years. The warranty is fully transferable and includes labor and parts for covered repairs. Extended warranty options up to 8 years are available through authorized distributors. All claims are managed via the Cingol Service Network with SLA-backed response times (within 48 hours for critical failures). |
| 5. How does Cingol ensure long-term serviceability and compatibility with future dental technologies? | Cingol chairs launched in 2026 feature modular architecture and open-protocol connectivity (HL7, DICOM, and IoT-ready). Firmware updates are delivered securely over-the-air (OTA), ensuring compatibility with evolving clinic management systems. The company adheres to a 15-year service lifecycle commitment, including access to technical documentation, retrofit kits, and upgrade paths. This future-proof design supports integration with AI-assisted diagnostics, augmented reality loupes, and robotic assistant systems. |
Note: Specifications and services are subject to change. For the latest technical documentation and distributor agreements, contact Cingol Global Sales Support at [email protected].
Need a Quote for Cingol Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


