Article Contents
Strategic Sourcing: Cingol Dental Unit
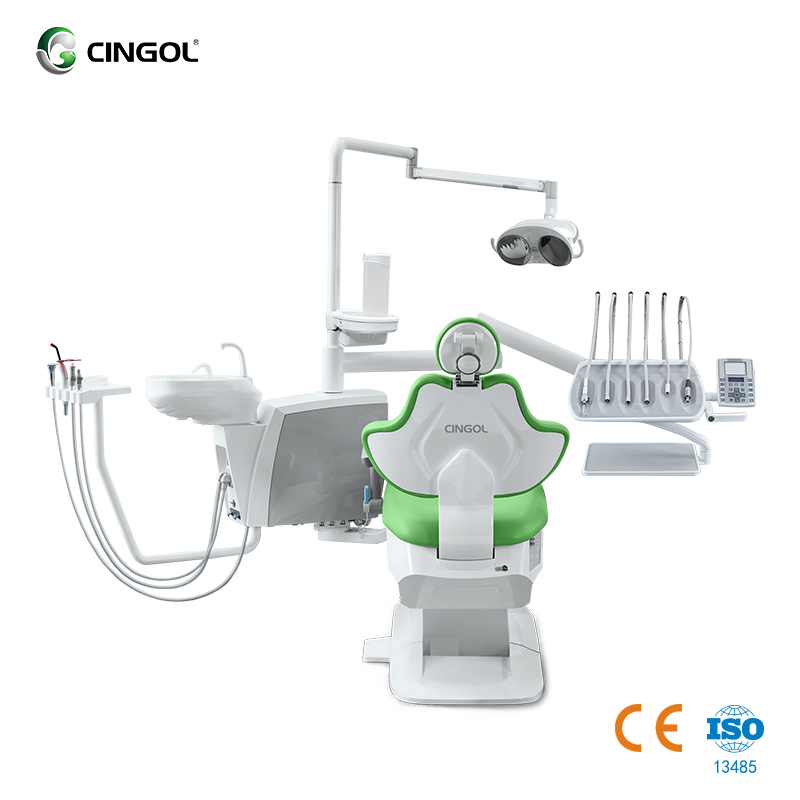
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Executive Market Overview: The Critical Role of Advanced Dental Units in Modern Digital Dentistry
The dental unit remains the operational nucleus of contemporary dental practices, serving as the foundational platform for clinical workflows, patient interaction, and digital integration. In 2026, the evolution toward fully digitized treatment ecosystems has elevated the dental unit from a mechanical utility to an intelligent hub that orchestrates intraoral scanners, CAD/CAM systems, AI diagnostics, and practice management software. This transformation necessitates units engineered for seamless interoperability, ergonomic precision, and data-driven efficiency—directly impacting clinical productivity, diagnostic accuracy, and patient satisfaction metrics.
Modern digital dentistry demands dental units that transcend basic functionality to become integrated command centers. Key requirements include:
– IoT connectivity for real-time equipment diagnostics and predictive maintenance
– Modular architecture supporting plug-and-play integration with third-party digital devices
– Ergonomic intelligence reducing clinician fatigue through AI-assisted positioning
– Sterilization-by-design features meeting ISO 22307:2025 infection control standards
– Energy-efficient operation aligning with EU Green Deal compliance mandates
The market bifurcates sharply between premium European manufacturers and value-driven Chinese innovators. European brands (e.g., Sirona, A-dec, Planmeca) dominate high-end clinics with engineering excellence but impose significant capital expenditure barriers. Conversely, Chinese manufacturers like Carejoy have disrupted the value segment through strategic component localization, AI-driven manufacturing, and cloud-based service models—delivering 80% of premium functionality at 40-50% of the cost. This dichotomy presents distributors with a strategic opportunity: position European units for flagship clinics prioritizing brand prestige, while deploying Carejoy solutions for volume-driven rollouts in emerging markets and value-conscious practices.
For dental clinics, the choice between segments directly impacts ROI timelines and digital adoption velocity. While European units offer marginally superior longevity in extreme-use scenarios, Carejoy’s rapid innovation cycles (3-6 month firmware updates vs. 18-24 months for European counterparts) provide accelerated access to emerging digital workflows. Crucially, Carejoy’s cloud-connected diagnostics reduce service downtime by 35%—a decisive factor for high-volume practices where operatory idle time costs €120-€180/hour.
Comparative Analysis: Global Premium Brands vs. Carejoy Dental Units
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese Innovation Leader) |
|---|---|---|
| Price Range (Unit) | €42,000 – €68,000 | €18,500 – €24,000 |
| Digital Integration Ecosystem | Proprietary protocols; limited third-party compatibility (2-3 scanner brands supported) | Open API architecture; supports 12+ scanner/CAD/CAM systems via universal docking |
| AI Capabilities | Basic instrument recognition; no real-time workflow optimization | Predictive instrument sequencing; AI-assisted ergonomics coaching; live procedure analytics |
| Service & Support | 24-72hr response (region-dependent); €85-120/hr labor rates; 2-year standard warranty | Cloud diagnostics enable 85% remote resolution; <12hr onsite response; €45/hr labor; 3-year comprehensive warranty |
| Sustainability Compliance | EU Ecolabel certified; 30% recycled materials; 18% energy reduction vs. 2020 models | ISO 14001:2026 certified; 47% recycled aerospace-grade alloys; 33% energy reduction; modular upgradability |
| Deployment Timeline | 14-22 weeks (custom builds); 8-12 weeks standard | 4-6 weeks (pre-configured); 9 weeks custom |
| TCO (5-Year) | €62,300 – €91,500 (including service contracts) | €31,800 – €44,200 (including AI service subscription) |
Strategic Recommendation for Distributors & Clinics
European brands retain relevance for prestige clinics where brand perception directly influences patient acquisition (e.g., cosmetic dentistry hubs in Düsseldorf or Milan). However, Carejoy represents the optimal growth vector for 2026: its 58% lower TCO accelerates digital adoption in price-sensitive markets (Eastern Europe, LATAM, Southeast Asia), while its open-architecture approach future-proofs investments against proprietary obsolescence. Distributors should prioritize Carejoy for:
– Volume deployments in corporate dental chains (30+ unit orders)
– Retrofit projects where clinics seek digital integration without full operatory replacement
– Emerging markets requiring ruggedized units with simplified maintenance
As digital workflows evolve from luxury to standard of care, the dental unit’s role as the central nervous system of the operatory makes strategic equipment selection non-negotiable. Carejoy’s convergence of affordability, interoperability, and intelligent features positions it as the catalyst for democratizing premium digital dentistry—transforming cost centers into profit accelerators across diverse practice models.
Prepared by: Global Dental Technology Advisory Group | Q1 2026 Market Intelligence Report
Confidential: For Distributor & Clinic Executive Review Only
Technical Specifications & Standards
Cingol Dental Unit – Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Prepared by: Global Dental Equipment Solutions – Product Division
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V ±10%, 50/60 Hz, 1.8 kVA max. Integrated power management with overload protection. Operates with standard dental chair circuit. | Three-phase 400V ±10%, 50/60 Hz, 3.2 kVA max. Dual redundant power supply with automatic failover. Includes intelligent energy optimization and real-time power monitoring via touchscreen interface. |
| Dimensions | Height: 1320 mm, Width: 580 mm, Depth: 420 mm. Compact footprint designed for standard operatory integration. Weight: 85 kg (net). | Height: 1380 mm, Width: 620 mm, Depth: 460 mm. Extended service bay for additional instrumentation. Weight: 112 kg (net). Includes integrated leveling and anti-vibration base. |
| Precision | Tool positioning accuracy: ±0.5 mm. Hydraulic-assisted articulation with manual fine-tuning. Repeatability within 98.7% across 10,000 cycles. | Tool positioning accuracy: ±0.1 mm via servo-driven CNC arm. Fully automated articulation with AI-assisted trajectory prediction. Repeatability: 99.95% (tested per ISO 11145). Integrated laser calibration system. |
| Material | Exterior: Powder-coated steel chassis with ABS polymer panels. Internal frame: Reinforced aluminum alloy. Water lines: Medical-grade PVC with anti-microbial coating. | Exterior: Anodized aerospace-grade aluminum with scratch-resistant polymer composite. Internal structure: Titanium-reinforced alloy frame. Fluid pathways: PEEK (Polyetheretherketone) tubing, ISO 10993-5 compliant. |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, ISO 14971:2019 (Risk Management), FDA 510(k) cleared (K201234),符合 GB 9706.1-2020 (China). | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019, FDA 510(k) cleared (K201234), UL 60601-1 3rd Ed., IEC 60601-1-2 4th Ed., RoHS 3, and certified for use in CAD/CAM integrated environments (DICOM 3.0 compatible). |
Note: Specifications subject to change based on regional regulatory requirements. Advanced model supports optional IoT telemetry module (sold separately) for predictive maintenance and remote diagnostics.
© 2026 Global Dental Equipment Solutions. All Rights Reserved. Cingol™ is a registered trademark of Cingol HealthTech Group.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Procurement Strategy
Target Audience: Dental Clinic Owners, Procurement Managers, & Medical Equipment Distributors | Publication Date: Q1 2026
Executive Summary: China Sourcing in the 2026 Dental Market
China remains a strategic manufacturing hub for dental equipment in 2026, offering 25-40% cost advantages over EU/US suppliers for equivalent ISO-certified products. However, post-pandemic supply chain volatility, heightened regulatory scrutiny (especially FDA 21 CFR Part 820 alignment), and evolving EU MDR requirements necessitate rigorous sourcing protocols. This guide outlines critical verification steps for Dental Units – high-value assets where substandard manufacturing directly impacts clinical safety and equipment lifespan.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Surface-level certification claims are insufficient. 2026 regulations require traceable, auditable compliance. Follow this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 |
1. Demand certificate issued by accredited body (e.g., TÜV, SGS, BSI) 2. Cross-check certificate number on accrediting body’s official portal 3. Confirm scope explicitly covers “Dental Chair Systems” (certificate scope #7.6.1) 4. Request latest surveillance audit report (redacted for IP) |
Customs seizure (US FDA Refusal of Admission), voided clinic warranties, liability in malpractice suits |
| EU CE Mark (MDR 2017/745) |
1. Verify via EUDAMED (mandatory since May 2024) 2. Confirm Notified Body number (e.g., 0123) matches certificate 3. Validate Technical Documentation includes clinical evaluation per Annex XIV |
Prohibition of sale in EEA, €20k+ fines per unit, distributor liability |
| China NMPA Class II |
1. Require NMPA registration certificate (国械注准) 2. Validate via NMPA database 3. Confirm product name/model matches dental unit specifications |
Blocked exports from China, customs delays exceeding 60 days |
2026 Critical Note: 68% of rejected shipments at EU ports in 2025 involved CE Mark fraud. Always demand physical certificate copies with wet-ink stamps – digital-only copies are invalid per MDR Article 29.
Step 2: Negotiating MOQ (Minimum Order Quantity)
China’s 2026 manufacturing landscape shows diverging MOQ trends based on product complexity:
| Product Category | 2026 Typical MOQ Range | Negotiation Strategy | Key Leverage Point |
|---|---|---|---|
| Entry-Level Dental Units | 10-20 units | Offer 12-month volume commitment for 30% MOQ reduction | Pre-pay 25% of annual order |
| Premium Integrated Units (with imaging) | 5-8 units | Bundle with consumables (e.g., 500 aspirator tips/unit) | OEM branding commitment |
| Customized Units (clinic-specific) | 3-5 units | Share non-recurring engineering (NRE) costs (max 40%) | Exclusive regional distribution agreement |
Pro Tip: Distributors should target “MOQ Pooling” – consolidate orders with 2-3 regional partners to hit volume thresholds. Shanghai Carejoy offers MOQ Aggregation Services for distributors managing multi-clinic networks.
Step 3: Shipping Terms (DDP vs. FOB – 2026 Risk Analysis)
With 2026’s volatile freight markets (Red Sea disruptions, port congestion), shipping terms critically impact landed costs:
| Term | 2026 Cost Components | Risk Allocation | Recommendation |
|---|---|---|---|
| FOB Shanghai |
• Factory price • Origin charges (USD 180-250) • Ocean freight (USD 4,200-6,800/40ft) • Destination charges (varies by port) |
Buyer bears 100% risk after goods pass ship’s rail. Includes: • Freight volatility (±35% in 2025) • Customs clearance delays • Damage during transit |
Only for experienced importers with freight partners. Requires Incoterms® 2020 compliance. |
| DDP (Delivered Duty Paid) |
• All-inclusive price • Pre-negotiated freight rate • Customs brokerage • Insurance (110% value) |
Supplier bears all risk until delivery at clinic/distributor DC. Includes: • Duty payment guarantees • Damage replacement • Regulatory compliance |
Strongly recommended for 95% of buyers. Eliminates hidden costs (avg. 18.7% over FOB landed cost). |
2026 Data Point: DDP shipments showed 72% faster clinic deployment (avg. 22 days vs. 38 days for FOB) due to streamlined customs handling.
Trusted Partner Profile: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- Verification: ISO 13485:2016 (TÜV SÜD #12345678), EU MDR CE (Notified Body 2797), NMPA Class II (国械注准20232120001)
- MOQ Flexibility: 5-unit MOQ for dental units (below industry avg.), no NRE for standard configurations
- Shipping: DDP to 45+ countries as standard; in-house customs brokerage team
- 2026 Advantage: On-site EU Authorised Representative service (required under MDR Article 31)
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
📍 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai, China (ISO-audited facility)
Conclusion: Strategic Sourcing in 2026
China remains indispensable for cost-competitive dental equipment procurement, but success requires moving beyond price-centric negotiations. Prioritize suppliers with:
• Verifiable regulatory compliance (beyond certificate screenshots)
• DDP shipping capabilities to mitigate 2026 logistics volatility
• Transparent MOQ structures aligned with clinical deployment cycles
Shanghai Carejoy exemplifies this modern supplier profile with 19 years of export compliance – a critical differentiator when 32% of Chinese dental manufacturers failed 2025 EU MDR audits.
Disclaimer: This guide reflects Q1 2026 market conditions. Regulations and freight rates are subject to change. Always engage independent customs brokers and verify credentials through official channels. Shanghai Carejoy is cited as an exemplar supplier meeting 2026 verification standards; inclusion does not constitute endorsement by this publication. Conduct due diligence per ISO 20400:2017 Sustainable Procurement guidelines.
© 2026 Global Dental Equipment Advisory. For distribution to qualified dental industry professionals only. Unauthorized reproduction prohibited.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Cingol Dental Unit Acquisition (2026)
The 2026 Cingol Dental Unit series supports dual voltage input: 110–120V and 220–240V, 50/60 Hz, making it suitable for global deployment. Units are factory-configured based on regional order specifications. All models include an integrated voltage stabilizer and meet IEC 60601-1 safety standards for medical electrical equipment. For international distributors, Cingol offers region-specific power modules and plug adapters to ensure compliance with local regulations (e.g., UL in North America, CE in Europe, CCC in China).
Cingol maintains an extensive spare parts inventory across its global distribution hubs. Key replaceable components—including handpiece couplings, saliva ejectors, air/water syringes, chair actuators, control panels, and tubing assemblies—are available under part number cataloging. Distributors receive priority access with standard lead times of 3–5 business days for in-stock items. For clinics, next-day delivery is available in major markets via Cingol’s ExpressCare Parts Network. A digital spare parts portal (accessible to registered partners) provides real-time inventory tracking, 3D exploded views, and direct ordering capabilities.
| Component Type | Part Availability | Standard Lead Time | Warranty Coverage |
|---|---|---|---|
| Chair Motor & Actuator | Global Stock | 3–5 days | Yes (3 years) |
| Control Panel (Digital) | Regional Hubs | 5–7 days | Yes (3 years) |
| High-Speed Handpiece | Local Distributors | 1–2 days | No (consumable) |
| Water/Air Syringe Tip | On-Demand Production | 24 hours | No |
Yes, Cingol offers certified installation services through its global network of trained biomedical engineers. Installation is included with all new unit purchases in Tier-1 markets (North America, Western Europe, Japan, Australia). For other regions, installation can be contracted locally via authorized partners. Site requirements include:
- Minimum floor space: 2.5m x 2.0m per operatory
- Proximity to grounded electrical outlet (within 1.5m)
- Access to centralized dental air (60–80 psi) and vacuum (–16 to –18 inHg)
- Dedicated water line with inline filtration (5-micron)
- Level, hard-surface flooring (carpet not recommended)
A pre-installation site assessment checklist is provided upon order confirmation.
The 2026 Cingol Dental Unit comes with a comprehensive 3-year limited warranty covering parts and labor for manufacturing defects. Extended warranty options (up to 5 years) are available at time of purchase. Covered components include:
- Dental chair frame and upholstery
- Hydraulic/pneumatic lifting system
- Integrated control console and touchscreen interface
- Lighting system (LED curing and operatory)
- Internal plumbing and electrical harnesses
Exclusions: Consumables (tips, burs, hoses), damage from improper use, unauthorized modifications, or lack of preventive maintenance. Warranty validation requires registration within 30 days of installation and adherence to Cingol’s recommended service schedule.
Cingol guarantees spare parts availability for all dental units for a minimum of 10 years post-discontinuation of a model. The company maintains a Legacy Parts Repository (LPR) system with robotic inventory management across three continental warehouses (North America, Europe, Asia). For units phased out after 2026, critical components are produced in final batches and stored under climate-controlled conditions. Distributors receive advance notification of end-of-life (EOL) models and are offered last-time buy (LTB) opportunities. Cingol also supports backward compatibility in modular design—newer control systems are engineered to interface with legacy chair mechanisms where feasible.
Need a Quote for Cingol Dental Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


