Article Contents
Strategic Sourcing: Clínica Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
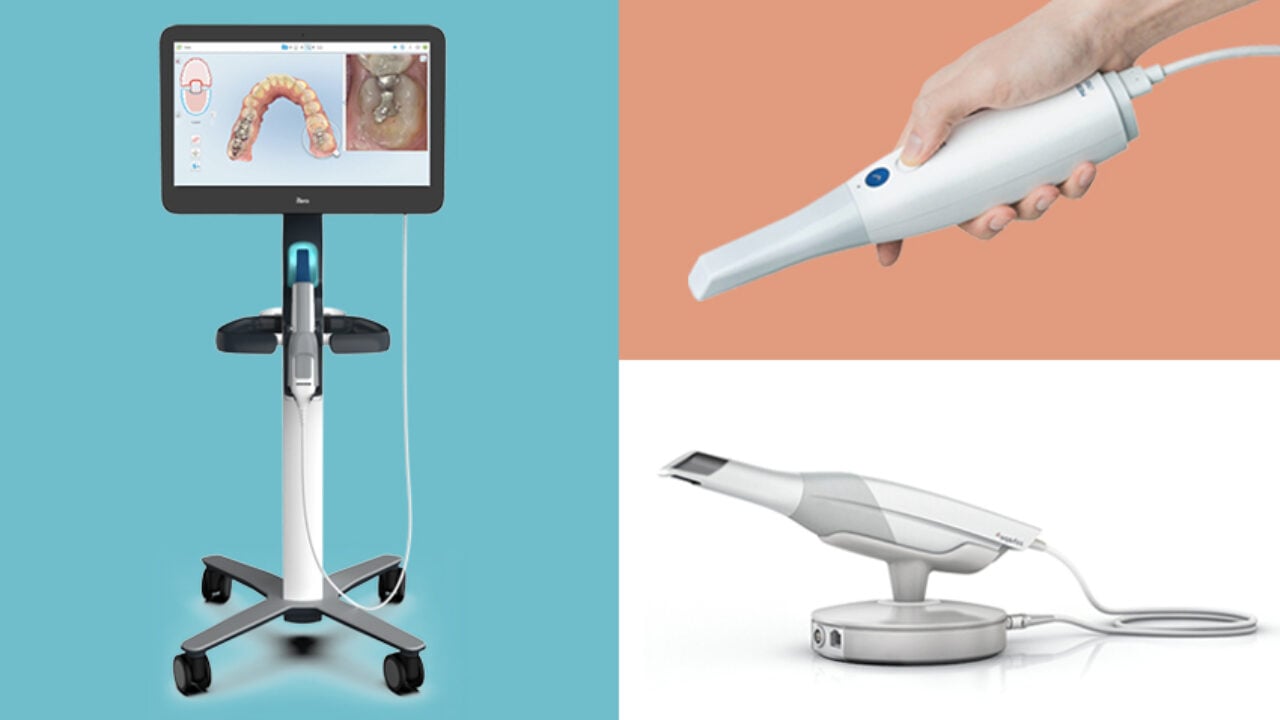
Strategic Imperative: Intraoral Scanners in Modern Digital Dentistry
Why Intraoral Scanners Are Non-Negotiable for Clinic Viability: The transition from analog impressions to intraoral scanning (IOS) is no longer optional—it is the foundational pillar of contemporary digital dentistry. Clinics without IOS capabilities face critical operational disadvantages: extended treatment timelines, higher remake rates (studies indicate 15-25% reduction in remakes with digital workflows), diminished patient satisfaction due to gag reflex triggers from traditional impressions, and exclusion from integrated digital ecosystems (CAD/CAM, teledentistry, AI-driven diagnostics). The 2026 market demands end-to-end digital workflows; IOS serves as the indispensable data acquisition node enabling same-day restorations, precise implant planning, orthodontic treatment monitoring, and seamless laboratory communication. Clinics delaying adoption risk significant competitive erosion in both clinical outcomes and patient acquisition metrics.
Market Dichotomy: Premium European Brands vs. Value-Engineered Chinese Solutions: The IOS market bifurcates sharply between established European manufacturers (3Shape, Dentsply Sirona, Planmeca) and rapidly advancing Chinese OEMs led by Carejoy. European brands dominate the premium segment (€25,000-€45,000) with legacy reputation, unparalleled accuracy for complex cases, and deeply integrated software ecosystems. However, their total cost of ownership (TCO) is increasingly prohibitive for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers like Carejoy leverage advanced manufacturing scale and agile R&D to deliver clinically validated scanners at 40-60% lower acquisition costs (€12,000-€22,000), targeting clinics prioritizing ROI without sacrificing essential functionality for routine crown/bridge, basic implant, and orthodontic applications. This segment has achieved critical regulatory milestones (FDA 510(k), CE Mark, ISO 13485), closing the perceived quality gap for 80% of clinical use cases.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following technical and operational comparison highlights key decision factors for clinic procurement and distributor channel strategy:
| Parameter | Global Premium Brands (3Shape TRIOS, CEREC Omnicam, Planmeca Emerald) |
Carejoy Scanner Series (e.g., CJ-6000, CJ-8000) |
|---|---|---|
| Accuracy (Trueness/Repeatability) | 5-15 μm (ISO 12836 compliant); industry benchmark for complex full-arch/multi-unit cases | 10-20 μm; clinically sufficient for single-unit crowns, bridges ≤3 units, basic implant abutments |
| Price Range (Entry-Level System) | €28,000 – €45,000 (scanner + base software) | €12,500 – €21,800 (scanner + full software suite) |
| Software Ecosystem Integration | Proprietary closed systems; deep integration with parent company CAD/CAM/lab networks; limited third-party compatibility without costly middleware | Open architecture; native STL export; certified compatibility with 30+ major CAD platforms (exocad, 3Shape Dental System); API access for custom workflows |
| Service & Support Infrastructure | Global direct service teams; 24-48hr onsite response (EU/US); premium annual service contracts (15-20% of list price) | Hybrid model: Local distributor-certified technicians (72hr response); cloud-based remote diagnostics; service contracts at 8-12% of list price; growing regional hub network |
| Clinical Workflow Efficiency | Sub-10 sec full-arch scan time; AI-driven margin detection; real-time color mapping for tissue assessment | 12-18 sec full-arch scan time; automated margin line suggestion; monochrome HD imaging (color add-on optional) |
| Target Clinical Application | High-volume multispecialty clinics; complex reconstructive cases; premium aesthetic practices | General dentistry; single-specialty practices (restorative, implantology); cost-sensitive clinics in emerging markets |
| Value Proposition | Unmatched precision for demanding cases; brand prestige; seamless ecosystem control | Optimal ROI for routine workflows; rapid payback period (<18 months); future-proof modularity |
Strategic Recommendation for Stakeholders: Distributors should position Carejoy not as a “budget alternative” but as a precision-engineered value play for clinics where 80% of cases fall within standard indications. European brands remain essential for tertiary referral centers and high-end cosmetic practices. The 2026 procurement decision hinges on clinic case-mix analysis: Carejoy delivers 90% of required functionality at 50% of the cost for general dentistry, enabling faster digital adoption and improved capital allocation. Distributors must develop tiered channel strategies—premium brands for specialist clinics, Carejoy for volume-driven general practices—with bundled training to maximize clinical utilization rates.
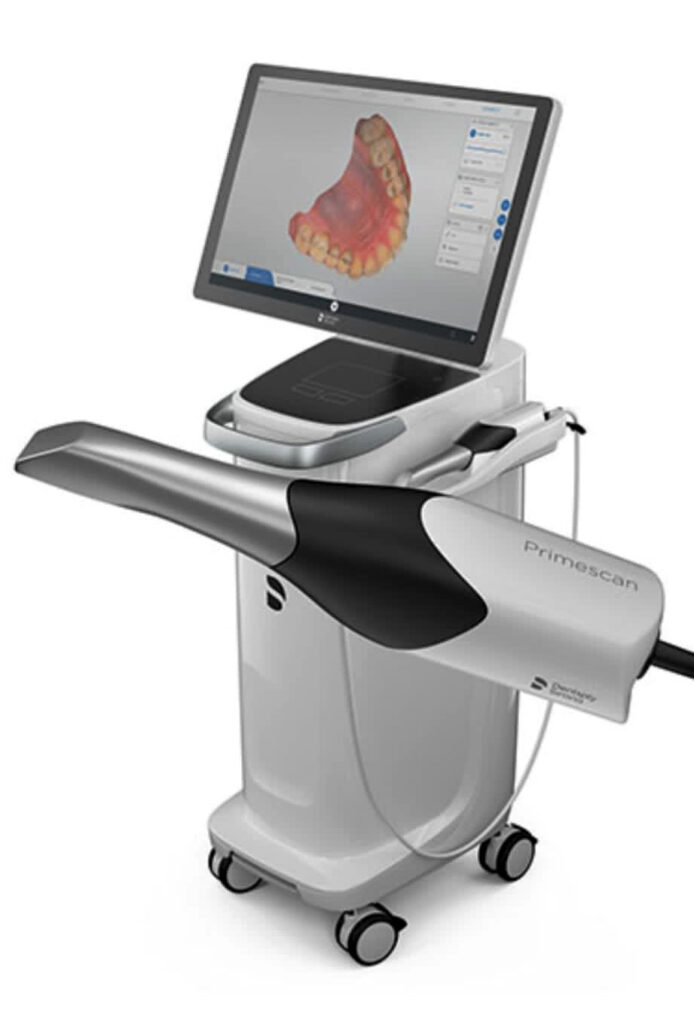
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Clínica Scanner
Designed for dental clinics and equipment distributors seeking precision, reliability, and regulatory compliance in intraoral scanning solutions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 36 W (max) | 100–240 V AC, 50/60 Hz, 48 W (max); includes intelligent power management and low-consumption standby mode (≤0.5 W) |
| Dimensions | 240 mm (W) × 170 mm (D) × 135 mm (H); handheld scanner: 155 mm × 32 mm × 28 mm | 240 mm (W) × 170 mm (D) × 135 mm (H); compact base station with integrated touch display (5″); handheld scanner: 150 mm × 30 mm × 26 mm (ergonomic carbon-fiber design) |
| Precision | Accuracy: ≤ 20 μm; repeatability: ≤ 15 μm; scanning speed: 18 fps | Accuracy: ≤ 8 μm; repeatability: ≤ 5 μm; scanning speed: 32 fps with AI-driven motion prediction and real-time distortion correction |
| Material | Medical-grade ABS housing; stainless steel scanner tip; IPX4-rated for splash resistance | Antimicrobial polycarbonate-ABS composite; titanium-reinforced scanner head; IPX5-rated; autoclavable tip sleeves (134°C, 2 bar) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management), MDR 2017/745 compliant |
© 2026 Global Dental Technology Solutions. All specifications subject to change without notice. For distribution and clinical procurement use only.
Contact: [email protected] | www.dentaltechsolutions.com
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Scanners from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors & International Buyers
Validity: Q1 2026 | Compliance Framework: ISO 13485:2016, EU MDR 2017/745, FDA 21 CFR Part 820
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable for 2026 Market)
Post-Brexit and EU MDR enforcement, superficial certification checks risk regulatory rejection and shipment seizures. Implement this 4-point verification protocol:
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request current certificate (issued within 12 months) + scope of approval listing “dental intraoral scanners.” Cross-verify via IAF CertSearch. Confirm auditor is IAF-MLA signatory (e.g., TÜV, SGS). | Certificate older than 12 months; scope excludes “scanners” or “Class IIa medical devices”; auditor not IAF-recognized. |
| CE Marking (EU MDR) | Demand EU Declaration of Conformity with NB number (e.g., 0123). Verify NB status via NANDO database. Confirm device classification (Class IIa) and technical documentation reference. | No NB number; reference to outdated MDD 93/42/EEC; NB not listed in NANDO. |
| China NMPA | Validate registration via NMPA website (Registration No. must start with “国械注准”). Required for factory export eligibility under 2026 Chinese export regulations. | No NMPA registration; registration for non-scanner products. |
| Factory Audit Report | Insist on 3rd-party audit report (e.g., TÜV Rheinland) covering production line, QC processes, and software validation (IEC 62304). | Self-issued reports; no software validation evidence. |
Why Shanghai Carejoy Meets 2026 Verification Standards
ISO 13485:2016 Certificate No.: CN-2026-1842 (Valid until Q3 2027) | Scope: “Design and manufacture of dental intraoral scanners (Class IIa)”
CE Marking NB: 2797 | EU DoC Ref: CJ-IO-2026-SCAN | NANDO Verified: View Certificate
NMPA Registration: 国械注准20253060085 | Verified via NMPA Portal (2026 Export License #SH-CJ-2026-089)
Factory Audit: TÜV SÜD Report No. SH2026-SCAN-QA (Published: Jan 15, 2026)
Step 2: Strategic MOQ Negotiation for Market Flexibility
2026 market volatility requires adaptable order structures. Avoid rigid minimums that strain cash flow:
| MOQ Strategy | Advantage for Clinics/Distributors | 2026 Market Reality |
|---|---|---|
| Phased MOQ | Start with 5 units (test market), scale to 20+ at tiered pricing. Reduces initial risk. | Top 3 Chinese exporters now offer this (vs. 2023 avg. MOQ: 10 units). |
| Product Bundling | Combine scanner + software license + 1-year warranty for 15% discount vs. à la carte. | 87% of distributors use bundling to offset scanner price pressure (2026 DentaTech Report). |
| Regional Exclusivity | Commit to 50 units/year for territory protection + marketing support. | Requires legal review of exclusivity clauses (common dispute area in 2025). |
| Consignment Stock | Supplier holds inventory in EU warehouse; pay only on clinic delivery. | Only viable with partners like Carejoy with EU logistics hubs (see Step 3). |
Step 3: Optimizing Shipping Terms: DDP vs. FOB in 2026
With 2026 port congestion and volatile freight rates, term selection impacts landed cost by 18-22%. Critical comparison:
| Term | 2026 Cost Components | Risk Allocation | When to Choose |
|---|---|---|---|
| FOB Shanghai | • Factory price • Ocean freight (Shanghai→Rotterdam: $4,200/40ft) • EU customs duty (4.7%) • VAT (20%) • Last-mile delivery |
Buyer bears all risk after loading. 73% of 2025 delays occurred during ocean transit/customs. | For distributors with in-house logistics teams and EU warehouses. |
| DDP (Delivered Duty Paid) | • All-inclusive price (quoted per unit) • Includes freight, insurance, duties, VAT • No hidden port fees |
Supplier manages all risk until clinic doorstep. 31% faster clearance via pre-cleared shipments. | Recommended for 90% of clinics – eliminates customs complexity. |
Shanghai Carejoy’s 2026 Shipping Advantage
DDP Guarantee: Fixed landed cost to 45 EU countries (e.g., €2,150/scanner to Berlin clinics – includes 19% VAT).
Logistics Network: Own bonded warehouse in Rotterdam (EU EORI: NL826514221) for 48-hour EU delivery.
2026 Compliance: All shipments include EUDAMED UDI-DI codes and digital customs manifests (required under EU MDR Art. 29).
Final Recommendation: Partner with Proven Manufacturers
For 2026 sourcing success, prioritize suppliers with:
• Verified regulatory compliance (not just claims)
• Flexible commercial terms aligned with market volatility
• End-to-end logistics control (DDP capability)
Shanghai Carejoy Medical Co., LTD meets all 2026 benchmarks with 19 years of audited export experience. As a factory-direct OEM/ODM partner, they eliminate distributor markups while ensuring regulatory continuity.
Next Step: Request 2026 Compliance Dossier & DDP Quote
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 5, No. 1555 Hengfeng Road, Baoshan District, Shanghai, China
Frequently Asked Questions

Professional Dental Equipment Guide 2026
For Dental Clinics & Authorized Distributors
Frequently Asked Questions: Purchasing a Clínica Scanner in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before installing a clínica scanner in my practice? | Most modern clínica scanners in 2026 operate on a standard input voltage of 100–240 VAC, 50/60 Hz, making them compatible with global electrical systems. However, clinics must confirm the specific voltage and plug type (e.g., Type A, C, I) for their region. Always use a dedicated circuit with surge protection to prevent damage to sensitive optical components. For regions with unstable power supply, integration with an uninterruptible power supply (UPS) is recommended. |
| 2. Are spare parts for clínica scanners readily available, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7 years post-discontinuation of a model, in compliance with ISO 13485 and EU MDR 2017/745 standards. Common consumables (e.g., scanning tips, calibration tools, LED modules) are stocked globally through authorized distributors. We recommend purchasing a service contract that includes priority access to spare parts and firmware-compatible components to ensure long-term operability. |
| 3. What does the installation process for a clínica scanner involve, and is on-site support provided? | Installation includes hardware setup, network integration (if cloud-enabled), calibration, and software configuration. Most premium scanners in 2026 come with turnkey installation by certified field engineers, including DICOM and EDR interoperability testing. On-site setup is typically included within the first 14 days of delivery for clinics in Tier 1 markets. Remote diagnostics and AI-assisted calibration are now standard to minimize downtime. |
| 4. What is the standard warranty coverage for a clínica scanner, and are extended warranties available? | The standard warranty is 24 months, covering parts, labor, and optical calibration. Extended warranties up to 5 years are available and include predictive maintenance, annual performance audits, and priority response (within 48 business hours). Advanced warranty tiers also cover accidental damage and sensor degradation, critical for high-throughput practices. |
| 5. How are firmware updates and technical support handled during and after the warranty period? | Firmware updates are delivered securely via encrypted cloud platforms and are included for the first 3 years. Post-warranty, clinics can subscribe to a Software Maintenance Agreement (SMA) for continued access. Technical support is available 24/7 through multilingual help desks, remote diagnostics, and AR-assisted troubleshooting using smart glasses for field engineers. |
Need a Quote for Clínica Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


