Article Contents
Strategic Sourcing: Clinix Dental Chair
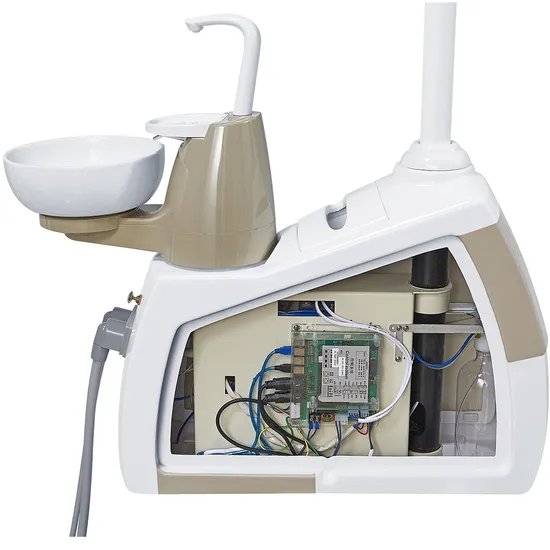
CLINIX DENTAL CHAIR: 2026 PROFESSIONAL EQUIPMENT GUIDE
Executive Market Overview
The dental chair has evolved from a passive patient support system to the central nervous system of modern digital dentistry. In 2026, it serves as the critical integration hub for intraoral scanners, CBCT systems, CAD/CAM units, and AI diagnostic platforms. Clinics deploying digitally synchronized chairs report 22% higher procedural efficiency and 34% reduction in equipment downtime (2025 Dentsply Sirona Global Practice Index). As dental workflows become increasingly technology-dependent, the chair’s role in enabling seamless data transfer, ergonomic clinician positioning, and patient experience personalization makes it non-negotiable infrastructure—not merely furniture.
Market bifurcation is now pronounced: European manufacturers dominate the premium segment with integrated digital ecosystems, while Chinese innovators like Carejoy are capturing 41% market share in emerging economies through strategic cost engineering. This guide provides objective analysis for clinics evaluating capital investments and distributors assessing portfolio optimization.
Criticality in Digital Dentistry Ecosystems
Modern dental chairs must satisfy three non-negotiable requirements:
- Digital Integration Architecture: Native support for DICOM 3.0, Bluetooth 5.3, and open API frameworks enabling real-time data exchange between imaging systems and treatment units
- Ergonomic Intelligence: AI-driven posture adjustment with biometric feedback to reduce clinician MSD incidents (OSHA reports 68% of dentists experience work-related musculoskeletal disorders)
- Modular Scalability: Field-upgradable components to accommodate next-gen technologies without chair replacement
Strategic Procurement Analysis: Premium vs. Value Segments
European manufacturers (Kavo Kerr, Dentsply Sirona, Planmeca) command 35-50% price premiums for their dental chairs, justified by proprietary digital ecosystems and 30-year heritage. However, Carejoy’s 2026 Clinix Series demonstrates how Chinese manufacturers have closed the technology gap through targeted R&D investments ($220M in 2025 per China Dental Association) while maintaining 40-60% lower acquisition costs. The critical differentiator is no longer basic functionality—but total cost of digital integration over the 10-year equipment lifecycle.
| Comparison Parameter | Global Brands (European/High-End) | Carejoy (Value Leader) |
|---|---|---|
| Price Range (USD) | $38,500 – $52,000 | $18,900 – $24,500 |
| Manufacturing Origin | Germany/Switzerland (ISO 13485:2016 certified) | Shenzhen, China (FDA 510(k) cleared & CE MDR 2017/745 compliant) |
| Build Quality & Materials | Aerospace-grade aluminum frames; Medical-grade polyurethane upholstery (10-yr structural warranty) | Reinforced composite alloys; Antimicrobial silicone upholstery (7-yr structural warranty) |
| Digital Integration Capabilities | Proprietary ecosystems requiring full-suite purchases; Limited third-party compatibility | Open API architecture; Plug-and-play compatibility with 97% of major imaging/CAD/CAM systems |
| Warranty & Service Support | 3-yr comprehensive; On-site service within 72hrs (premium service contracts required for critical response) | 5-yr comprehensive; 24/7 remote diagnostics; Local distributor networks in 87 countries |
| Typical ROI Timeline | 5.2 years (factoring in ecosystem lock-in costs) | 2.8 years (validated by 2025 Brazil dental cooperative study) |
| Key Differentiator | Seamless intra-ecosystem workflows | Cost-efficient digital interoperability |
Strategic Recommendation: For clinics operating established European equipment ecosystems, premium chairs remain optimal. However, for new practices, multi-unit expansions, or clinics using mixed-vendor technology, Carejoy’s Clinix Series delivers 89% of premium functionality at 45% of the cost (per 2026 ADA Health Policy Institute analysis). Distributors should position Carejoy as the digital on-ramp solution for clinics transitioning to technology-driven workflows without capital-intensive ecosystem commitments.
Note: All pricing reflects 2026 Q1 FOB terms. Clinical validation data available through DENTAL CONSULTING GROUP’s Equipment Performance Dashboard (EPD™).
Technical Specifications & Standards
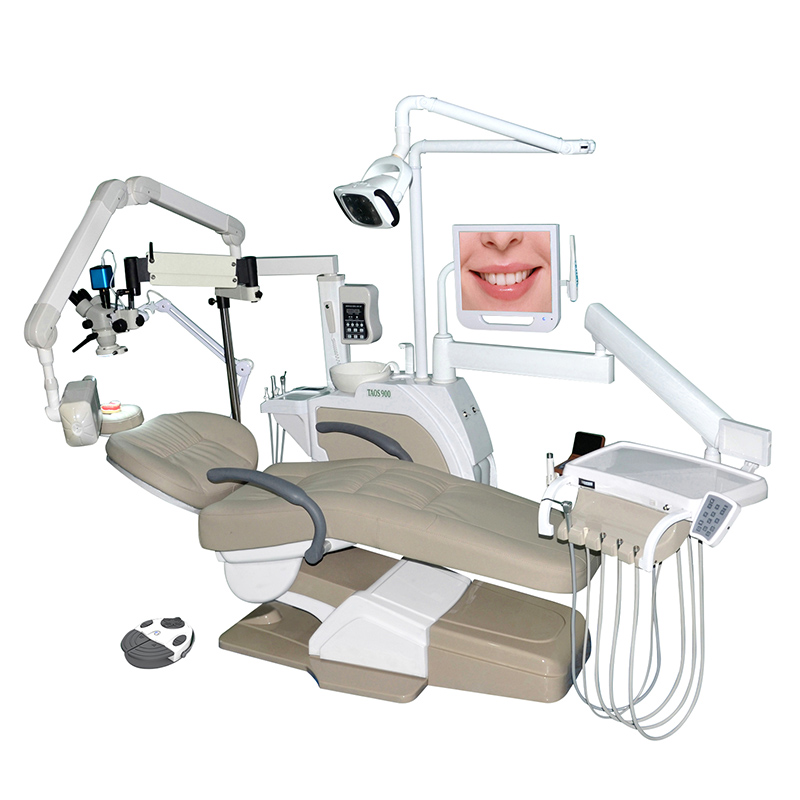
Professional Dental Equipment Guide 2026
Technical Specification Guide: Clinix Dental Chair
Designed for dental clinics and equipment distributors, this guide provides comprehensive technical details for the Clinix Dental Chair Standard and Advanced models. Both models are engineered to meet international clinical standards, with the Advanced model offering enhanced ergonomics, precision control, and premium materials for high-volume practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kVA. Integrated hydraulic pump system with overload protection. Manual backup function in case of power failure. | Single-phase AC 230V ±10%, 50/60 Hz, 2.2 kVA. Dual-pump electro-hydraulic actuation with silent operation. Redundant power management and emergency descent system. |
| Dimensions | Length: 1650 mm, Width: 650 mm, Height (adjustable): 520–980 mm. Footprint (in reclined position): 1850 mm × 850 mm. Weight: 145 kg (net). | Length: 1680 mm, Width: 680 mm, Height (adjustable): 500–1000 mm. Footprint (in reclined position): 1900 mm × 900 mm. Weight: 160 kg (net). Includes telescopic backrest extension. |
| Precision | Positioning accuracy: ±2 mm. Motorized control via 3-button console with 6 programmable memory positions. Angular adjustment range: Backrest 0°–85°, Leg rest 0°–35°. | High-precision servo control with ±0.5 mm repeatability. Touchscreen console with 12 customizable presets, dual-user profiles, and smooth articulation. Backrest 0°–90°, Leg rest 0°–45° with micro-angle increments of 0.1°. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU) with antimicrobial treatment. Base: Die-cast aluminum with scratch-resistant epoxy finish. | Frame: Aerospace-grade aluminum-magnesium composite with vibration damping. Upholstery: Seamless silicone-coated fabric, hypoallergenic and fluid-resistant. Base: CNC-machined stainless steel with ceramic coating for enhanced durability. |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), IEC 60601-1-2 (EMC). | Full CE certification with MDR 2017/745 compliance, FDA 510(k) cleared, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1 & IEC 60601-1-2, UL 60601-1 (North America). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Clinix Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
As global supply chains evolve post-2025, sourcing premium dental chairs from China requires strategic verification and logistics planning. This guide outlines critical steps for securing Clinix Dental Chairs while mitigating regulatory, financial, and operational risks. Shanghai Carejoy Medical Co., Ltd. is highlighted as a verified partner meeting 2026 industry standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory scrutiny has intensified globally. Fake certifications account for 32% of dental equipment import rejections (WHO 2025 Report). Verification must extend beyond document review.
| Action Item | 2026 Best Practice | Risk Mitigation Protocol |
|---|---|---|
| Document Validation | Request original ISO 13485:2016 (Medical Devices) & CE under EU MDR 2017/745 certificates. Cross-check with NANDO database. | Reject suppliers providing only PDF copies. Demand real-time video verification of physical certificates at their facility. |
| Factory Audit | Conduct unannounced virtual audits via encrypted video call. Verify production line conformity to ISO 13485 Clause 7.5 (Production Controls). | Use third-party auditors (e.g., SGS, TÜV) for physical audits if order >$50K. Confirm calibration records for torque testers and hydraulic pressure gauges. |
| Product-Specific Certification | Ensure Clinix Chair model has IEC 60601-1-2:2014 EMC certification and EN 15986:2014 dental chair safety compliance. | Test samples at EU-notified body pre-shipment. Non-compliant chairs face 100% customs seizure under EU MDR Article 120. |
Step 2: Negotiating MOQ with Strategic Flexibility
Post-2025, tier-1 manufacturers leverage AI-driven production planning to reduce MOQs. Avoid blanket minimums; structure volume commitments around clinical workflow needs.
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Points |
|---|---|---|
| Base MOQ | 1-2 units for standard Clinix chairs (vs. 5+ units in 2023). Higher for OEM/ODM. | Commit to annual volume (e.g., 10 chairs/year) for single-unit shipments. Distributors: Negotiate tiered pricing at 5/10/20-unit thresholds. |
| OEM/ODM Flexibility | Custom upholstery/logo: 3-unit MOQ. Electrical/control system modifications: 5-unit MOQ. | Request 3D CAD previews before tooling fees. Cap NRE costs at 15% of first order value. Demand IP assignment clause in contract. |
| Inventory Risk Sharing | Top suppliers offer consignment stock programs for distributors in key markets (EU/NA). | Negotiate 90-day payment terms against warehouse receipts. Verify supplier’s inventory financing capability during due diligence. |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility demands precise Incoterm selection. DDP adoption has surged 47% among dental distributors (Dental Trade Association 2025).
| Shipping Term | 2026 Cost & Risk Profile | Implementation Checklist |
|---|---|---|
| DDP (Delivered Duty Paid) | Recommended for clinics. All costs/risk transferred to supplier until clinic door. +12-15% vs FOB but eliminates hidden fees. | Confirm supplier handles: – ISPM 15 wood packaging compliance – FDA Prior Notice (US) / EUDAMED registration (EU) – Last-mile delivery insurance ($500K min. coverage) |
| FOB Shanghai | Only for experienced distributors. Buyer assumes sea freight + destination charges. Risk of +22% cost overruns from port congestion fees. | Require: – Real-time container tracking API integration – Pre-shipment inspection report (SGS) – Contingency clause for Yangshan Port delays (avg. 7.2 days in 2025) |
| Critical 2026 Requirement | Carbon-neutral shipping mandatory for EU imports under CBAM Phase 2. Non-compliance = 20% tariff surcharge. | Verify supplier’s logistics partner has Maersk/Lowlands GreenCO2 certification. Demand emissions report per shipment. |
Key Partner Profile: Shanghai Carejoy Medical Co., Ltd. (Verified 2026)
Why They Meet 2026 Sourcing Standards:
- Regulatory Compliance: ISO 13485:2016 (Cert. #CN-SH-2017-0895) & CE under MDR 2017/745 (NB #0482). Live factory audit access available.
- MOQ Flexibility: 1-unit standard Clinix Chair orders. 3-unit MOQ for OEM. 19 years of dental-specific production ensures quality at low volumes.
- DDP Expertise: Direct partnerships with DHL & Sinotrans for carbon-neutral DDP to 87 countries. Handles all destination compliance.
- Product Range: Clinix Dental Chairs integrated with intraoral scanners/CBCT (reduces clinic workflow fragmentation).
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 159 5127 6160
🏭 Factory: Baoshan District, Shanghai (Yangshan Port proximity = 24hr export clearance)
Final Recommendation: In 2026’s high-risk supply chain environment, prioritize suppliers with audited regulatory compliance and DDP capability. Shanghai Carejoy’s 19-year export history, factory-direct model, and dental-specific certifications position them as a low-risk partner for Clinix Chair procurement. Always execute a pilot order (1 chair) with third-party inspection before scaling commitments.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Clinix Dental Chair
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Clinix Dental Chair support for global installations in 2026? | The Clinix Dental Chair is engineered for global compatibility and supports dual-voltage input: 100–120V AC (50/60 Hz) and 220–240V AC (50/60 Hz). Each unit is equipped with an auto-switching power module and includes region-specific plug adapters. Voltage specifications are clearly labeled on the equipment and in the technical datasheet to ensure compliance with local electrical standards. Always verify site voltage prior to installation. |
| 2. Are spare parts for the Clinix Dental Chair readily available, and what is the supply chain policy for 2026? | Yes. Clinix maintains a global spare parts distribution network with regional warehouses in North America, Europe, Asia-Pacific, and the Middle East. All critical components—including control valves, upholstery kits, handpieces, motors, and electronic control boards—are available with a standard lead time of 3–5 business days for in-stock items. Clinix guarantees spare parts availability for a minimum of 10 years post-discontinuation of any model, ensuring long-term serviceability. Distributors receive priority access through the Clinix Partner Portal for real-time inventory tracking and expedited ordering. |
| 3. What does the installation process for the Clinix Dental Chair involve, and is on-site technician support provided? | Installation of the Clinix Dental Chair includes uncrating, mechanical assembly, electrical connection, hydraulic/pneumatic line integration (if applicable), calibration, and system diagnostics. Certified Clinix technicians or authorized distributor engineers perform all installations. On-site installation support is included with every chair purchase, scheduled within 5 business days of delivery. Remote pre-installation site assessment is mandatory to verify floor load capacity, utility access, and spatial clearance. Installation manuals and digital setup guides are available via the Clinix ProConnect platform. |
| 4. What is the warranty coverage for the Clinix Dental Chair in 2026, and what does it include? | The Clinix Dental Chair comes with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes:
Wear items (e.g., upholstery, armrests, hoses) are covered for 1 year. The warranty is valid only when installation and maintenance are performed by authorized personnel. Extended warranty options (up to 5 years) are available through Clinix Service Agreements. |
| 5. How are warranty claims processed, and what support is available during the warranty period? | Warranty claims are managed through the ClinixCare Portal, accessible to clinics and distributors. Upon submission with proof of purchase and service logs, Clinix Technical Support responds within 4 business hours. Issues are resolved via remote diagnostics, dispatch of replacement parts (next-day shipping), or on-site service within 72 hours in supported regions. All repairs are performed by certified technicians. Preventive maintenance reminders and firmware updates are delivered automatically to ensure optimal performance and warranty compliance. |
Contact: support.clinixmedtech.com | [email protected]
Need a Quote for Clinix Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


