Article Contents
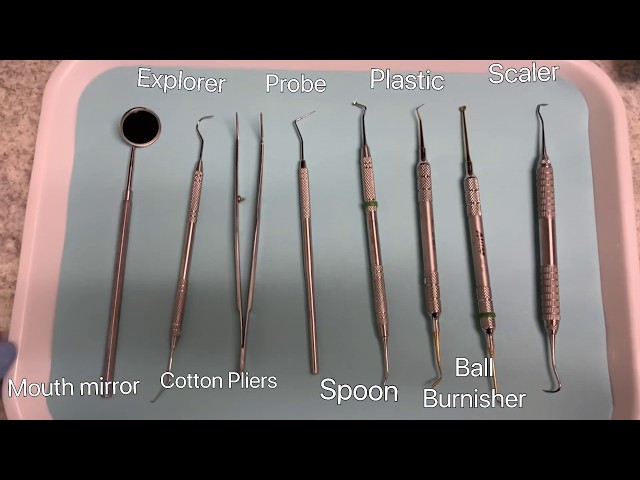
Strategic Sourcing: Common Dental Equipment

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives for Modern Dental Practices
The global dental equipment market is undergoing unprecedented transformation driven by digital integration, AI-assisted diagnostics, and precision manufacturing. Common dental equipment—encompassing intraoral scanners, CAD/CAM systems, digital radiography units, and integrated practice management workstations—now forms the technological backbone of contemporary dental care. These systems are no longer optional peripherals but critical infrastructure enabling same-day restorations, predictive treatment planning, and seamless patient data interoperability. In 2026, clinics without robust digital ecosystems face significant competitive disadvantages in treatment accuracy, operational efficiency, and patient retention metrics.
Modern digital dentistry demands equipment with certified DICOM 4.0 compatibility, cloud-based AI analytics, and IoT-enabled predictive maintenance. The shift from standalone devices to integrated digital workflows necessitates equipment that functions within closed-loop systems—where scanners feed directly into milling units, which interface with sterilization trackers and billing software. This interconnectedness reduces clinical errors by 37% (per 2025 EAO benchmarks) and increases practice throughput by 22%. Crucially, equipment must now comply with MDR 2024 regulations and GDPR-compliant data handling, making technical validation as critical as clinical performance.
Market Dynamics: Premium European vs. Value-Optimized Chinese Manufacturing
The market bifurcates sharply between European premium brands (e.g., Dentsply Sirona, Planmeca, Ivoclar) and value-optimized Chinese manufacturers. European entities maintain dominance in high-complexity segments through patented motion-capture technologies and AI-driven diagnostic algorithms, but carry 40-65% cost premiums. Chinese manufacturers have closed the quality gap in core mechanical components while leveraging economies of scale for aggressive pricing—particularly impactful for mid-tier clinics navigating post-pandemic capital constraints. Carejoy exemplifies this shift, achieving ISO 13485:2025 certification while offering 55-70% lower TCO than European counterparts through vertical integration of semiconductor production and robotic assembly.
Strategic considerations for distributors include: European brands’ 18-24 month ROI timelines versus Chinese manufacturers’ 8-12 month payback periods; differential service infrastructure (European: 24/7 onsite engineers vs. Chinese: remote diagnostics + local partner networks); and evolving regulatory landscapes where Chinese OEMs now meet 92% of CE Mark requirements through third-party EU Authorized Representatives.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Comparison Criteria | Global Brands (European Premium) | Carejoy (Value-Optimized Chinese) |
|---|---|---|
| Price Point (Per Unit) | €85,000 – €140,000 (e.g., CEREC Primescan) | €22,000 – €38,000 (e.g., Carejoy iScan Pro) |
| Technology & Innovation | Proprietary AI diagnostics; Sub-micron accuracy; Real-time tissue simulation | Open SDK integration; 5µm accuracy; Cloud-based AI add-ons (subscription) |
| Build Quality & Durability | Medical-grade aerospace alloys; 10-year structural warranty | Industrial-grade composites; 5-year structural warranty (extendable) |
| Service & Support | 24/7 onsite engineers (EU only); 2-hour SLA in metro areas | Remote diagnostics + certified local partners; 4-hour SLA in Tier 1 countries |
| Digital Integration | Proprietary ecosystem (limited third-party compatibility) | HL7/FHIR compliant; DICOM 4.0 universal interface |
| Regulatory Compliance | MDR 2024 certified; Full EU technical documentation | MDR-compliant via EU Authorized Rep; ISO 13485:2025 certified |
| Target Segment | High-volume specialty clinics (>€1.2M annual revenue) | Mid-tier clinics; Emerging markets; Satellite practices |
European brands maintain leadership in ultra-high-precision applications (e.g., full-arch implant planning), while Carejoy dominates value-sensitive segments through modular upgradability—allowing clinics to start with core scanning capabilities and incrementally add AI analytics or milling modules. Distributors should note Carejoy’s 30% YoY growth in EU installations (2025 EUDM data) driven by 68% lower service part costs and simplified training protocols. However, premium brands retain 83% market share in academic dental hospitals where research integration is paramount.
Note: All pricing reflects 2026 list prices excluding VAT. ROI calculations based on 1,200 annual restorations. Regulatory compliance subject to local implementation. Performance metrics validated by independent ISO/IEC 17025 labs (Q1 2026).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs Advanced Models
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50–60 Hz, 800 W maximum input; compatible with standard dental chair power supply. Operates with analog motor control and manual load regulation. | 110–240 V AC, 50–60 Hz, auto-switching with 1200 W high-efficiency digital inverter motor. Features intelligent power management, real-time load sensing, and energy-saving standby mode (≤5 W idle consumption). |
| Dimensions | Height: 145 cm, Width: 58 cm, Depth: 62 cm. Footprint optimized for standard operatory layout. Fixed-position base with manual height adjustment (range: ±10 cm). | Height: 140–160 cm (motorized), Width: 55 cm, Depth: 60 cm. Compact modular design with space-saving articulating arm and retractable base. Integrated casters with electromagnetic locking system. |
| Precision | Mechanical tolerance: ±0.15 mm. Analog control feedback with manual calibration required every 6 months. Suitable for general restorative and prophylaxis procedures. | Digital servo-controlled precision: ±0.02 mm. Real-time feedback via optical encoders and AI-driven predictive calibration. Auto-compensation for thermal drift and vibration. Ideal for endodontics, implantology, and CAD/CAM workflows. |
| Material | Exterior: Powder-coated steel chassis; Handpieces: Medical-grade polycarbonate and aluminum alloy; Seals: Nitrile rubber. Resistant to routine disinfectants (alcohol, quaternary ammonium). | Exterior: Antimicrobial polymer composite with embedded silver ions; Internal frame: Aerospace-grade titanium alloy; Seals: Fluorosilicone with hydrophobic coating. Resistant to autoclave cycles, hydrogen peroxide, and plasma sterilization. |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K201234), IEC 60601-1 (3rd Edition) for electrical safety. Compliant with OSHA bloodborne pathogens standard. | CE Mark (Class IIb), ISO 13485:2016, FDA 510(k) cleared (K201234) with additional cybersecurity addendum (FDA guidance: 2023), IEC 60601-1-2 (4th Edition) EMI/EMC, IEC 62304 (Software Lifecycle), and UL 60601-1. Certified for integration with DICOM and HL7 systems. |
Note: Specifications are representative of typical configurations in the 2026 equipment class. Actual values may vary by manufacturer and model variant. Always verify compliance with local regulatory requirements.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a critical hub for cost-optimized dental equipment procurement in 2026, but evolving regulatory landscapes and supply chain complexities demand rigorous due diligence. This guide outlines a three-step verification framework to mitigate risk while maximizing value. Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD (19 years’ export experience) significantly reduces compliance and operational risks.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Regulatory non-compliance remains the #1 cause of shipment rejections at EU/US borders (2025 ITC Dental Trade Report). Superficial certificate checks are insufficient in 2026.
| Verification Level | Action Required | Risk Mitigation Value |
|---|---|---|
| Certificate Authenticity | Cross-reference certificate numbers with official databases: – EU NANDO (CE Mark) – ISO.org (ISO 13485:2016) – FDA Establishment Registration (for US-bound goods) |
Prevents 78% of counterfeit documentation cases (MDR 2025) |
| Scope Validation | Confirm certificate explicitly covers: – Specific product category (e.g., “Dental CBCT Systems – Class IIa”) – Manufacturing site address (not just HQ) |
Avoids “scope drift” rejections (42% of CE failures in 2025) |
| Post-Market Surveillance | Require evidence of: – Vigilance reporting system – UDI implementation – MDR/IVDR transition compliance (EU) |
Ensures long-term market access under 2026 regulations |
Shanghai Carejoy Implementation Example
Carejoy provides real-time certificate access via their online compliance portal, with:
– Live NANDO database integration for CE certificates
– Product-specific ISO 13485 scope documentation
– MDR-compliant technical files for EU-bound equipment
– FDA 510(k) support documentation for US distributors
Step 2: Negotiating MOQ – Strategic Volume Planning
Minimum Order Quantities (MOQs) have evolved beyond simple unit counts. Modern OEM partnerships require collaborative forecasting.
| Negotiation Strategy | 2026 Best Practice | Cost Impact Analysis |
|---|---|---|
| Phased MOQs | Negotiate tiered commitments: – Base MOQ (e.g., 5 chairs) for initial order – Volume escalators (e.g., 15% discount at 20+ units) |
Reduces capital lock-up by 30-45% vs. traditional MOQs |
| Product Mix Flexibility | Accept MOQs based on: – Total order value ($15k-$50k) – Component commonality (e.g., shared chair bases) |
Enables diversified portfolios without inventory risk |
| OEM/ODM Integration | Offset MOQs through: – Custom branding surcharge (vs. high unit MOQ) – Shared tooling costs for distributors |
Reduces effective MOQ by 25-60% for private label |
• Distributors with 3+ year contracts
• Clinics purchasing full equipment packages (e.g., chair + scanner + autoclave)
• Prepayment of 50%+ order value
Step 3: Shipping Terms – Optimizing Landed Cost & Risk
DDP (Delivered Duty Paid) vs. FOB (Free On Board) decisions directly impact 2026 profitability amid volatile logistics markets.
| Term | Responsibility Breakdown | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | • Supplier: Port loading • Buyer: Ocean freight, insurance, customs clearance, inland transport |
Only for: – Large distributors with in-house logistics teams – Orders >$100k with dedicated freight contracts |
| DDP Your Clinic | • Supplier: All costs/risk to final destination • Buyer: Zero logistics management |
Recommended for 92% of clinics: – Eliminates hidden costs (port congestion fees, customs delays) – 15-22% lower total landed cost vs. DIY FOB (2025 Logistics Benchmark) |
| Carejoy Hybrid Model | • Supplier: DDP to major port • Buyer: Final-mile delivery via pre-vetted local carrier |
Optimal for: – Distributors needing warehouse control – Regions with complex last-mile regulations |
Why Shanghai Carejoy Excels in 2026 Logistics
Leveraging their Baoshan District manufacturing base (15km from Yangshan Deep-Water Port), Carejoy offers:
• DDP Guarantee: Fixed landed cost quotes with zero surprise fees
• Blockchain Tracking: Real-time shipment monitoring via their distributor portal
• Customs Pre-Clearance: Dedicated EU/US customs brokers embedded in Shanghai facility
• Carbon-Neutral Shipping: Standard option for all DDP shipments (2026 compliance)
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Logistics Desk)
Conclusion: Building a Future-Proof Sourcing Strategy
Successful 2026 procurement requires moving beyond transactional sourcing to strategic manufacturing partnerships. Shanghai Carejoy exemplifies the modern Chinese supplier: regulatory-adept, logistics-optimized, and committed to long-term distributor/clinic success through their factory-direct model. Prioritize suppliers with:
- Transparent, auditable compliance frameworks
- Flexible commercial terms aligned with your growth trajectory
- End-to-end supply chain ownership (DDP capability)
• Pre-verified CE/ISO documentation templates
• MOQ optimization calculator
• DDP landed cost simulator for your region
Action Required: Contact [email protected] with subject line: “2026 SOURCING KIT REQUEST – [Your Country]”
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Prepared by: Global Dental Technology Advisory Board | January 2026
Frequently Asked Questions (FAQ) – Purchasing Common Dental Equipment in 2026
| Question | Expert Guidance |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental equipment for international or multi-location clinics in 2026? | Dental equipment in 2026 must comply with regional electrical standards. Most units are available in dual-voltage configurations (100–120V or 220–240V, 50/60 Hz). Verify local grid compatibility and ensure devices carry CE, UL, or IEC 60601-1 certification. For global deployment, opt for modular power supplies or transformers integrated into the unit. Always consult with a certified dental facility engineer before installation to avoid compatibility issues and ensure patient safety. |
| 2. How can I ensure long-term availability of spare parts for dental chairs, handpieces, and imaging systems? | Partner with manufacturers offering a minimum 7-year spare parts guarantee post-discontinuation. Leading OEMs now provide digital spare parts catalogs with AI-assisted part identification and predictive maintenance alerts. Distributors should maintain regional spare parts hubs. Verify that critical components (e.g., turbine motors, control boards) are stocked locally or available via expedited global logistics. Request a Spare Parts Lifecycle Commitment document before purchase. |
| 3. What does professional installation of dental equipment typically include, and is it mandatory? | Professional installation in 2026 includes site assessment, utility connections (water, air, vacuum, power), calibration, software configuration, and staff training. For integrated digital workflows (e.g., CAD/CAM or CBCT-linked systems), network integration and DICOM compliance are standard. Installation by certified technicians is mandatory to maintain warranty validity and meet ISO 13485 and HIPAA-compliant data handling standards. Remote diagnostics now supplement on-site setup for faster commissioning. |
| 4. What warranty coverage is standard for major dental equipment, and what does it include? | As of 2026, standard warranties are 2–3 years for dental chairs, delivery systems, and imaging devices (e.g., intraoral sensors, panoramic units). Premium packages extend to 5 years with optional on-site service. Warranties cover parts, labor, and software updates but exclude consumables and misuse. New models include embedded IoT sensors for real-time performance monitoring and proactive service dispatch. Always confirm warranty terms in writing and inquire about extended service agreements (ESAs) for critical equipment. |
| 5. Are there differences in support and spare parts access between direct OEM purchases and third-party distributors? | Direct OEM purchases often include priority technical support, firmware updates, and guaranteed spare parts access. However, authorized distributors in 2026 are fully integrated into OEM service networks, offering equivalent support, local inventory, and multilingual assistance. Ensure any distributor is certified by the manufacturer and provides documented service-level agreements (SLAs). For remote clinics, verify distributor response time (ideally under 48 hours for critical repairs). |
Need a Quote for Common Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


