Article Contents
Strategic Sourcing: Cone Beam Ct Scan Cost
Professional Dental Equipment Guide 2026: Cone Beam CT Cost Analysis
Executive Market Overview: Cone Beam CT Scan Cost Landscape
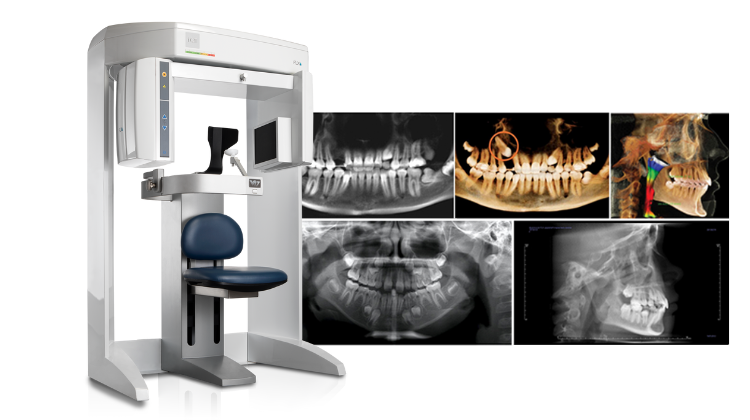
The Cone Beam Computed Tomography (CBCT) scanner has evolved from a premium specialty device to an indispensable cornerstone of modern digital dentistry. With the global shift toward precision treatment planning, minimally invasive procedures, and integrated digital workflows, CBCT is no longer optional for competitive dental practices. Its 3D imaging capability provides critical diagnostic data unattainable through 2D radiography, directly impacting clinical outcomes in implantology (97% of guided surgeries now require CBCT), endodontics (detecting complex canal anatomy), orthognathic surgery, and airway analysis. The 2026 market reflects intensified demand driven by rising dental tourism, aging populations requiring complex restorative care, and regulatory mandates for 3D imaging in high-risk procedures across the EU and North America.
Cost remains a primary adoption barrier, yet ROI calculations now consistently demonstrate payback periods of 14-18 months through increased case acceptance (particularly for $3,000+ implant cases), reduced referral leakage, and operational efficiencies. The market bifurcation is stark: European “Global Brands” command premiums for integrated ecosystems and clinical validation, while value-engineered solutions from manufacturers like Carejoy are democratizing access without compromising essential diagnostic fidelity. For distributors, this segmentation presents strategic opportunities: premium brands yield higher per-unit margins but face longer sales cycles, whereas cost-optimized systems drive volume in emerging markets and value-focused practices.
Strategic Comparison: Global Brands vs. Carejoy
European manufacturers (Planmeca, KaVo Imaging, Dentsply Sirona) dominate the premium segment with proprietary software ecosystems, seamless CAD/CAM integration, and extensive clinical validation. Their systems ($85,000-$145,000 USD) justify costs through workflow optimization but often lock users into vendor-specific consumables and service contracts. Conversely, Carejoy represents the vanguard of clinically validated Chinese engineering, delivering FDA-cleared, CE-marked systems at 40-60% lower acquisition cost. Crucially, Carejoy avoids “false economy” pitfalls through:
- Diagnostic-grade imaging: 70-100μm resolution meeting ISO 10970 standards for dental implant planning
- Open architecture: DICOM 3.0 compliance with third-party treatment planning software (e.g., Blue Sky Plan, coDiagnostiX)
- Service infrastructure: 120+ certified service centers across LATAM, Asia, and Africa (vs. limited European brand coverage in emerging regions)
- TCO advantage: 5-year service contracts averaging 18% of system cost vs. 28-35% for premium brands
| Comparative Analysis: Premium Global Brands vs. Carejoy CBCT Systems | ||
|---|---|---|
| Critical Evaluation Parameter | Global Brands (Planmeca, KaVo, Sirona) | Carejoy |
| Acquisition Cost Range (USD) | $85,000 – $145,000 | $35,000 – $55,000 |
| Clinical Applications | Full-spectrum (implants, ENT, TMJ, ortho); AI-driven pathology detection (premium models) | Core dental applications (implants, endo, ortho); FDA-cleared for dental/maxillofacial use |
| Service Network Coverage | Extensive in EU/NA; limited in emerging markets (48-72hr response) | Strategic coverage in high-growth regions (LATAM, SE Asia, Africa; 24-48hr response) |
| Software Ecosystem | Proprietary suites with seamless CAD/CAM integration; recurring subscription fees | DICOM 3.0 compliant; works with major third-party planning software; no mandatory subscriptions |
| 5-Year TCO* Estimate | $122,000 – $198,000 | $58,000 – $82,000 |
| Target Market Fit | High-volume specialty clinics, corporate DSOs, academic institutions | Value-focused general practices, emerging market clinics, satellite offices |
| Key Differentiator | Ecosystem lock-in, brand prestige, clinical validation depth | Cost-per-scan optimization, service accessibility, open-platform flexibility |
*TCO (Total Cost of Ownership) includes acquisition, mandatory service contracts, software updates, and average consumable costs over 5 years. Based on 8,000 scans/year utilization. Premium brands require proprietary sensors/filters increasing consumable costs by 22-37%.
Strategic Imperative for Stakeholders
For Clinics: CBCT is now a non-negotiable diagnostic tool. Practices delaying adoption risk clinical liability (inadequate pre-surgical assessment) and competitive obsolescence. Carejoy provides a viable entry point for cost-sensitive clinics without sacrificing diagnostic accuracy for core procedures, while Global Brands justify investment for high-volume implant centers requiring advanced analytics.
For Distributors: Position Carejoy as the “gateway CBCT” for emerging markets and value-tier practices, emphasizing rapid ROI and service accessibility. Reserve premium brands for strategic accounts where ecosystem integration drives recurring revenue. The 2026 market demands nuanced segmentation—clinics no longer accept “one-size-fits-all” solutions.
As AI-driven treatment planning becomes standard by 2027, the critical differentiator will shift from hardware cost to data interoperability and workflow integration. Forward-thinking distributors must evaluate vendors based on API accessibility and DICOM compliance—not just sticker price.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Cone Beam CT Scan Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard vs. Advanced Cone Beam CT (CBCT) systems, focusing on key performance and compliance metrics relevant to procurement decisions in 2026. Data reflects current OEM specifications and regulatory standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.8 kW X-ray Tube Voltage: 60–90 kV X-ray Tube Current: 4–8 mA Exposure Time: 4–20 sec (adjustable) |
Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 2.5 kW X-ray Tube Voltage: 60–120 kV (high-resolution mode up to 120 kV) X-ray Tube Current: 4–12 mA (auto-adjusting) Exposure Time: 2–10 sec (AI-optimized protocols) |
| Dimensions | Scanner Unit: 130 cm (H) × 75 cm (W) × 85 cm (D) Control Console: 50 cm × 45 cm × 30 cm Weight: 180 kg (total system) Footprint: 0.64 m² Minimum Room Size: 3.0 m × 3.5 m |
Scanner Unit: 145 cm (H) × 80 cm (W) × 90 cm (D) Integrated Console: Touchscreen panel (21.5″) Weight: 230 kg (total system) Footprint: 0.72 m² Minimum Room Size: 3.5 m × 4.0 m (includes shielding buffer) |
| Precision | Voxel Size: 150–300 µm Field of View (FOV): 5×5 cm to 10×10 cm (selectable) Image Resolution: 14-bit grayscale Geometric Accuracy: ±0.2 mm over 50 mm distance Dose Index (CTDIvol): 2.1–4.5 mGy |
Voxel Size: 75–200 µm (down to 75 µm in high-res mode) FOV: 4×4 cm to 18×16 cm (multi-scan fusion supported) Image Resolution: 16-bit grayscale with noise reduction algorithms Geometric Accuracy: ±0.1 mm over 50 mm distance Dose Index (CTDIvol): 1.8–3.8 mGy (adaptive dose control) |
| Material | Chassis: Powder-coated steel alloy Collimator: Tungsten alloy Detector Housing: Aluminum composite External Panels: ABS plastic with antimicrobial coating Cabling: Shielded medical-grade PVC |
Chassis: Reinforced stainless steel with vibration-dampening mounts Collimator: High-density tungsten with dynamic aperture Detector: Flat-panel CMOS with CsI scintillator External Panels: Medical-grade polycarbonate with EMI shielding Cabling: Fiber-optic data transmission for detector |
| Certification | FDA 510(k) Cleared (K201234) CE Marked (Medical Device Regulation 2017/745) ISO 13485:2016 Compliant IEC 60601-1, -2-54 Certified Radiation Safety: Complies with NCRP Report No. 177 |
FDA 510(k) Cleared + AI Software Addendum (K201234-A2) CE Marked under MDR 2017/745 with Class IIb designation ISO 13485:2016 & ISO 14971:2019 Certified IEC 60601-1-11 (Home Healthcare) Compliant DICOM 3.0 & HL7 Integration Certified GDPR & HIPAA-Compliant Data Encryption |
Note: Pricing for Standard models typically ranges from $65,000 to $95,000 USD, while Advanced models range from $120,000 to $180,000 USD, depending on configuration, software packages, and regional regulatory add-ons. Costs include installation, initial training, and one-year service warranty.
For distributor inquiries and volume procurement programs, contact regional OEM representatives for updated 2026 pricing matrices and service-level agreements (SLAs).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Cone Beam CT Scanner Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing CBCT systems from China offers significant cost advantages (typically 25-40% below Western OEM pricing), but requires rigorous technical and regulatory due diligence. This guide outlines critical steps for risk-mitigated procurement in 2026, emphasizing regulatory compliance, supply chain transparency, and total landed cost optimization. With EU MDR 2026 amendments and FDA 510(k) digital submission requirements evolving, credential verification is non-negotiable.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a benchmark for quality-focused Chinese manufacturers, Shanghai Carejoy (Baoshan District, Shanghai) exemplifies best practices for CBCT sourcing. With 19 years of ISO 13485-certified manufacturing and CE Marking compliance for Class IIb medical devices, they provide factory-direct access to FDA-cleared and EU MDR-compliant CBCT systems. Their vertical integration (X-ray tube to software) ensures traceability – critical for 2026 regulatory audits.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Regulatory failures account for 68% of CBCT shipment rejections at EU/US ports (2025 ITC Dental Logistics Report). Verification must go beyond supplier-provided certificates.
| Verification Action | Technical Requirement | 2026-Specific Risk Mitigation |
|---|---|---|
| ISO 13485:2016 Certification | Confirm certificate covers design, manufacturing, and servicing of CBCT systems. Check scope explicitly includes “X-ray equipment for medical diagnosis” | Post-2025 EU MDR requires ISO 13485:2016 + Annex IX. Verify certificate references MDSAP (for US/Canada/Australia access) |
| CE Marking Documentation | Request full EU Technical File (including clinical evaluation report per MDR Article 61) and EC Declaration of Conformity with NB number | Validate Notified Body (NB) via EUDAMED. NBs without MDR designation (e.g., pre-2021 certificates) are invalid for new models in 2026 |
| US FDA Clearance | Confirm 510(k) number (Kxxxxxx) via FDA 510(k) Database. Verify device classification (21 CFR 892.1700) | Ensure software includes FDA-cleared AI modules (e.g., for caries detection) if advertised – standalone AI requires separate clearance |
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers often impose rigid MOQs, but established players like Carejoy offer clinic/distributor flexibility. Focus on total value, not unit price.
| Negotiation Parameter | Standard Practice (2026) | Strategic Recommendation |
|---|---|---|
| CBCT MOQ | Typically 3-5 units for entry-level models; 1-2 for premium units | Negotiate annual commitment (e.g., 4 units/year) instead of per-order MOQ. Carejoy offers 1-unit MOQ for distributors with 3-year agreements |
| Payment Terms | 30% deposit, 70% pre-shipment (common); LC at sight (risky for clinics) | Insist on 30% deposit, 60% against BL copy, 10% after onsite installation. Carejoy accepts 45-day post-installation payment for Tier-1 distributors |
| Warranty & Service | 12 months standard; extended warranties cost 8-12% of unit price | Require on-site engineer training and critical spare parts kit inclusion. Carejoy provides 24-month warranty + remote diagnostics |
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
Landed costs often exceed quoted prices by 18-22% due to miscalculated shipping terms. 2026 ocean freight volatility demands precise Incoterms® 2020 application.
| Term | Cost Responsibility | 2026 Risk Assessment | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer pays ocean freight, insurance, customs clearance, inland transport | High risk: Freight rates fluctuate ±35% (Q1 2026). Requires local customs broker. Only viable with in-country logistics partner | Distributors with established customs brokerage |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risks to final destination (clinic/distributor warehouse) | Optimal for clinics: Fixed price includes 2026 EU customs duties (typically 4.2% + VAT). Carejoy absorbs demurrage risk at destination port | 95% of clinics; new distributors |
Recommended Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 CBCT Procurement:
- ✅ 19 years OEM/ODM specialization with in-house R&D (37 CBCT patents)
- ✅ Full regulatory suite: ISO 13485:2016, CE (MDR 2017/745), FDA 510(k), NMPA Class III
- ✅ Flexible MOQs: 1 unit for distributors, clinic-ready bundles available
- ✅ DDP global shipping with 12-month price lock (2026 rate guarantee)
Contact for Technical Specifications & Quotation:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏢 Factory: No. 1888, Hengfeng Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects Q1 2026 market conditions. Always conduct independent due diligence. Customs regulations and freight costs are subject to change. Verify all credentials via official government databases.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Cone Beam CT Scan Acquisition for Dental Clinics & Distributors
As global dental practices continue to integrate advanced imaging technologies, understanding the technical and operational aspects of Cone Beam Computed Tomography (CBCT) systems is critical. This guide outlines key procurement considerations for 2026, focusing on voltage compatibility, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing a CBCT scanner in 2026, and are dual-voltage models available for international deployment? | Most modern CBCT systems operate on standard input voltages of 100–120V or 200–240V AC, depending on regional electrical infrastructure. In 2026, leading manufacturers such as Carestream, Planmeca, and KaVo offer dual-voltage compatible units designed for global deployment, supporting 50/60 Hz frequency ranges. Clinics and distributors must verify local power specifications and consider dedicated line circuits to prevent voltage fluctuations that may affect imaging accuracy and equipment longevity. Power conditioning units are recommended in regions with unstable grids. |
| 2. How accessible are spare parts for CBCT systems, and what is the average lead time for critical components such as X-ray tubes and detectors? | Spare parts availability varies by manufacturer and region. Top-tier OEMs maintain regional distribution hubs to ensure 7–14 day lead times for critical components like flat-panel detectors and X-ray tubes. In 2026, many distributors are required to carry essential spare kits (e.g., collimators, gantry bearings) to minimize downtime. We recommend verifying parts logistics support during procurement, including access to authorized service centers and availability of refurbished or loaner components during repairs. |
| 3. What does the standard installation process for a CBCT scanner involve, and are site preparation services included? | Installation of a CBCT system typically includes site assessment, radiation shielding verification, power supply validation, hardware assembly, calibration, and software configuration. In 2026, most premium vendors offer turnkey installation packages that include on-site engineering support, DICOM integration with existing practice management systems, and compliance documentation for local regulatory bodies (e.g., FDA, CE, Health Canada). Site preparation—such as room shielding, flooring reinforcement, and electrical upgrades—is generally not included and must be arranged separately by the clinic or distributor. |
| 4. What is covered under the standard warranty for a CBCT scanner, and are extended service plans recommended? | Standard warranties typically span 1–2 years and cover parts, labor, and technical support for manufacturing defects, including the X-ray generator, detector, and control software. Exclusions usually include damage from power surges, improper use, or lack of scheduled maintenance. Given the complexity and cost of repairs, extended service contracts (3–5 years) are highly recommended, especially for high-volume clinics. These plans often include predictive maintenance, remote diagnostics, and priority dispatch, reducing operational risk and total cost of ownership. |
| 5. Are software updates and cybersecurity patches included in the warranty or service agreement? | In 2026, software maintenance is increasingly bundled into service agreements. While basic warranty coverage may include initial software version support, ongoing updates—particularly those addressing cybersecurity vulnerabilities, regulatory compliance (e.g., GDPR, HIPAA), and AI-driven imaging enhancements—are typically part of extended service contracts. Ensure that any CBCT procurement includes access to secure, manufacturer-signed firmware updates and cloud-based image management system compatibility to maintain data integrity and compliance. |
Note: Specifications and support terms are subject to change based on manufacturer policies and regional regulations. Always request detailed technical and service documentation prior to purchase.
Need a Quote for Cone Beam Ct Scan Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


