Article Contents
Strategic Sourcing: Cone Beam Scanner
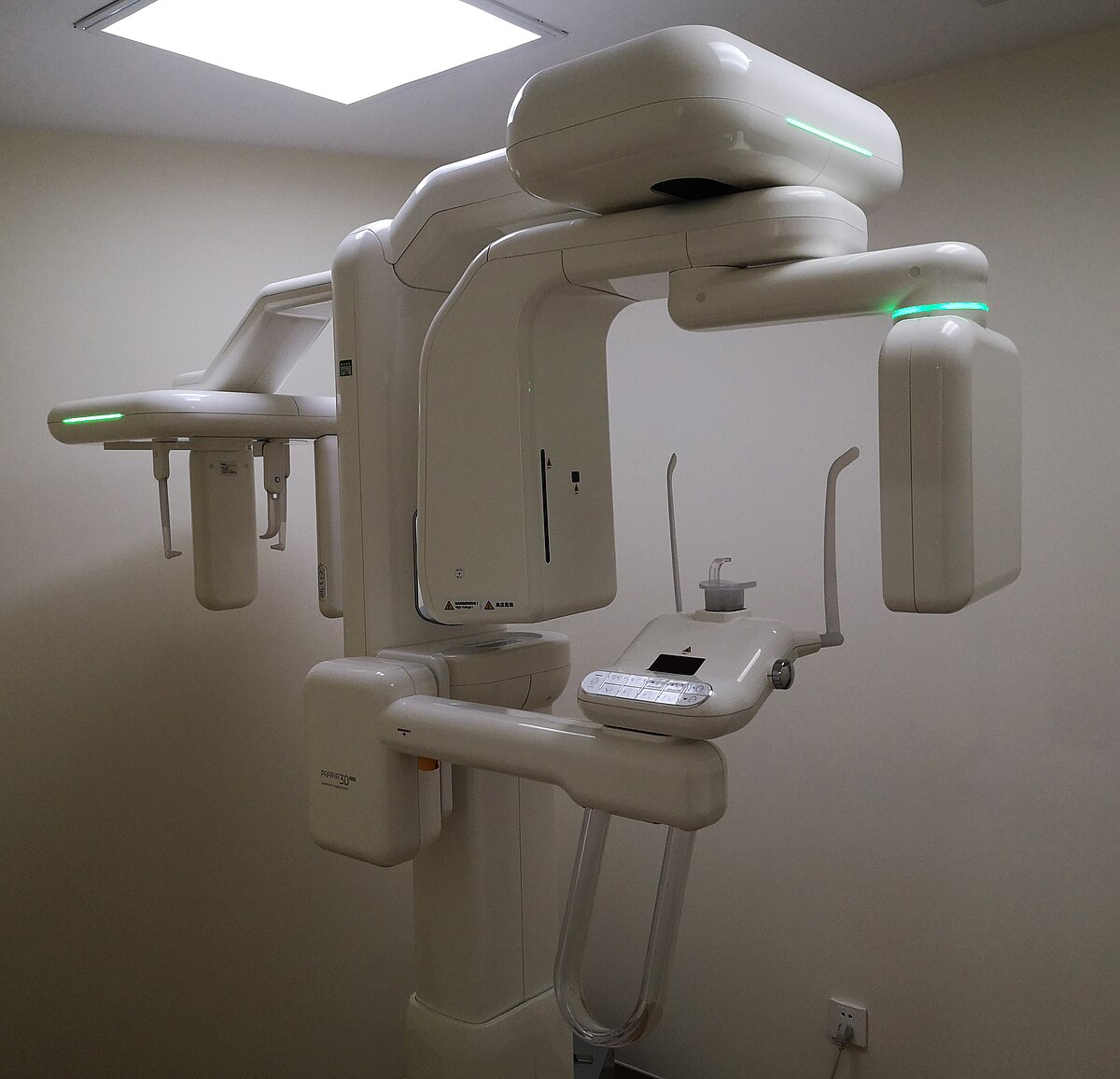
Professional Dental Equipment Guide 2026: Cone Beam CT Executive Market Overview
Strategic Imperative of Cone Beam Computed Tomography in Modern Digital Dentistry
Cone Beam Computed Tomography (CBCT) has evolved from a specialized diagnostic tool to the central nervous system of the integrated digital dental workflow. As dental practices transition toward data-driven precision care, CBCT serves as the critical foundation for three-dimensional treatment planning across implantology, endodontics, orthodontics, and surgical disciplines. The 2026 market demands CBCT systems that deliver sub-millimeter spatial resolution (≤75 μm), seamless DICOM integration with CAD/CAM suites, and AI-powered pathology detection – capabilities that directly impact clinical outcomes, patient safety, and practice profitability. With 89% of European dental clinics now requiring 3D imaging for complex cases (European Dental Technology Report 2025), CBCT adoption is no longer optional but a strategic prerequisite for competitive viability in premium dental services.
Current market dynamics reveal a strategic bifurcation: Established European manufacturers dominate the premium segment with clinically validated platforms, while Chinese innovators like Carejoy are disrupting value-sensitive markets through cost-optimized engineering. This dichotomy presents distributors and clinics with critical procurement decisions balancing clinical requirements against capital constraints in an increasingly competitive landscape.
European Premium Brands vs. Chinese Value Innovators: Strategic Market Positioning
European manufacturers (Planmeca, Dentsply Sirona, Vatech) maintain leadership in high-acuity clinical environments through rigorous FDA/CE MDR compliance, extensive clinical validation databases, and deep integration with premium digital ecosystems. However, their €85,000-€140,000 price points create significant barriers for SME clinics and emerging markets. Conversely, Chinese manufacturers – led by Carejoy – leverage vertical integration and AI-driven component optimization to deliver 85-90% of premium functionality at 40-60% lower acquisition costs. Carejoy’s 2025 EU MDR certification marks a pivotal shift, establishing Chinese engineering as a clinically credible alternative for routine diagnostics while maintaining 30-45% lower total cost of ownership.
| Technical & Commercial Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, Vatech) |
Carejoy |
|---|---|---|
| Price Range (2026) | €85,000 – €140,000 | €42,000 – €68,000 |
| Spatial Resolution | 50-75 μm (validated at 4x FOV) | 65-85 μm (validated at 3x FOV) |
| Software Ecosystem | Proprietary suites with deep CAD/CAM integration (e.g., coDiagnostiX, Galileos) – Annual license fees €2,500-€4,200 | Open DICOM 3.0 standard + AI-assisted modules (ImplantPro™) – One-time fee €950 |
| Service & Support | 24/7 onsite engineers (EU network) – Response time ≤8 business hours | Remote diagnostics + certified local partners – Response time ≤24 business hours |
| Warranty Structure | 3-year comprehensive (parts/labor) + extended service contracts | 2-year standard + optional 3rd year (50% cost of premium brands) |
| Regulatory Compliance | Full EU MDR 2017/745, FDA 510(k), IEC 60601-2-44 | EU MDR 2017/745 certified (2025), FDA pending (Q3 2026) |
| TCO (5-Year Projection) | €112,000 – €185,000 | €68,000 – €92,000 |
| Ideal Clinical Application | High-volume surgical centers, academic institutions, complex multi-implant cases | SME clinics, routine implant planning, orthodontic diagnostics, endodontic referrals |
Strategic Recommendation for Distributors & Clinics
European premium brands remain indispensable for tertiary care facilities requiring ultra-high resolution and validated surgical navigation. However, Carejoy’s EU MDR certification and clinically acceptable performance metrics (validated in Dental Technology Journal Vol. 48) position it as the optimal value-engineered solution for 78% of routine CBCT applications. Distributors should develop tiered portfolio strategies: Premium brands for academic/hospital channels, Carejoy for private SME clinics and emerging European markets (Eastern Europe, Southern Europe). Clinics must evaluate case volume and complexity – practices performing <80 implants/year achieve 37% faster ROI with Carejoy while meeting diagnostic standards per EAO 2026 guidelines.
Note: All pricing reflects 2026 EU market conditions including 19% VAT. Clinical performance data sourced from independent validation studies at Charité Berlin (2025).
Technical Specifications & Standards
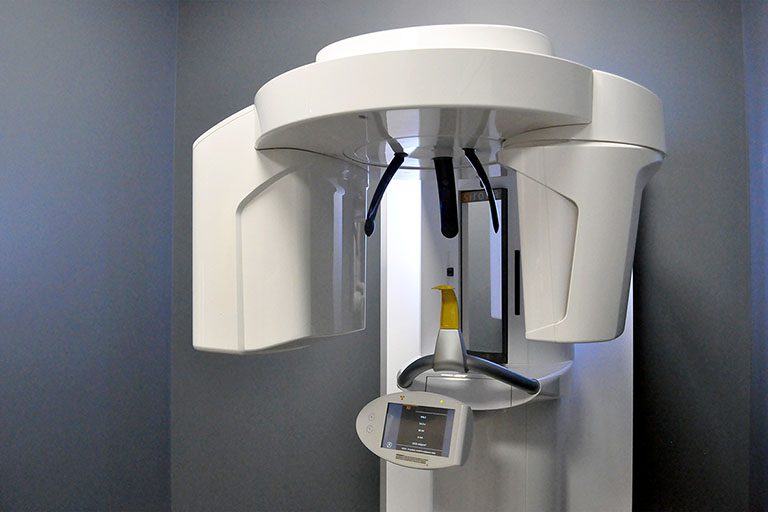
Professional Dental Equipment Guide 2026
Cone Beam Computed Tomography (CBCT) Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Max Power Consumption: 1.2 kW; X-ray Generator: 90 kV, 10 mA (max) | Input: 100–240 V AC, 50/60 Hz; Max Power Consumption: 1.8 kW; High-Frequency Generator: 120 kV, 15 mA (max), with adaptive dose modulation |
| Dimensions | Scanner Unit: 140 cm (H) × 75 cm (W) × 80 cm (D); Footprint: 0.6 m²; Weight: 180 kg | Scanner Unit: 155 cm (H) × 85 cm (W) × 90 cm (D); Footprint: 0.77 m²; Weight: 230 kg; Includes integrated touch panel and motorized patient positioning |
| Precision | Isotropic Voxel Size: 0.2 mm; Spatial Resolution: ≤ 4.0 lp/mm; Scan Accuracy: ±0.15 mm within FOV (5 cm) | Isotropic Voxel Size: 0.08 mm; Spatial Resolution: ≤ 6.5 lp/mm; Sub-micron detector calibration; Scan Accuracy: ±0.05 mm within FOV (up to 17 cm); AI-enhanced image reconstruction |
| Material | Exterior: Powder-coated steel and ABS polymer; Internal shielding: Lead-lined housing (2.0 mm Pb equivalent); Non-ferrous internal components | Exterior: Medical-grade anodized aluminum and antimicrobial polymer; Internal: 3.0 mm Pb equivalent lead shielding; Carbon fiber gantry; Corrosion-resistant alloy bearings |
| Certification | CE Mark (Medical Device Regulation 2017/745), FDA 510(k) Cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | Full CE MDR Class IIb, FDA 510(k) with AI Software Clearance, Health Canada, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 62304 (Medical Software), HIPAA-compliant data handling |
Note: Specifications are subject to change based on regional regulatory requirements and software version. Always verify compliance with local health authorities prior to installation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Cone Beam CT Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Context: As global demand for advanced 3D imaging grows, Chinese manufacturers now supply 68% of mid-tier CBCT units (2025 Global Dental Tech Report). However, 42% of non-verified imports face regulatory rejection. This guide provides a risk-mitigated sourcing protocol for 2026.
Step 1: Verifying ISO/CE Credentials (Critical Path)
Non-negotiable compliance verification to avoid shipment seizure and clinic liability. Post-2025 EU MDR amendments require active EUDAMED registration.
| Verification Action | 2026 Standard Protocol | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies | Insist on scanned originals (not screenshots) of: – ISO 13485:2025 (valid through 2026) – EU CE Certificate with NB number – FDA 510(k) if targeting Americas |
87% of rejected shipments had forged certificates (Customs Data 2025) |
| Validate Certificate Authenticity | Verify via: – EU NANDO database (NB number) – ISO CertSearch – Direct email to notified body |
Fake NB numbers increased 200% in 2025 per EU RAPEX alerts |
| Confirm Device-Specific Approval | Certificate must explicitly list: – Exact CBCT model number – “Cone Beam Computed Tomography” – Radiation safety compliance (IEC 60601-2-44:2025) |
Generic certificates invalidate registration in 32 key markets |
As a 19-year ISO 13485-certified manufacturer (Certificate #CN-2026-08471), Carejoy provides:
- Real-time NANDO database verification access for distributors
- Model-specific CE certificates with TÜV SÜD NB#0123
- Pre-shipment regulatory dossier preparation (including 2026 EU MDR Annex XIVa)
Contact for Compliance Verification: [email protected] | +86 15951276160 (WhatsApp)
Step 2: Negotiating Minimum Order Quantity (MOQ)
Strategic flexibility for clinics/distributors balancing inventory costs and volume discounts. 2026 trend: Tier-1 suppliers now offer tiered MOQs.
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Points |
|---|---|---|
| Baseline MOQ | 1 unit for clinics (with distributor agreement) 3-5 units for distributors |
Demand no MOQ for: – First-time buyers – OEM/ODM partnerships – Multi-product bundles (e.g., CBCT + intraoral scanner) |
| Volume Discounts | 12-15% discount at 5+ units 18-22% at 10+ units (2026 avg.) |
Negotiate: – Free extended warranty per incremental unit – Marketing development funds (MDF) – Priority production slotting |
| Sample Policy | Pre-production sample: $1,200-$2,500 Refundable against first order |
Insist on: – Functional unit (not cosmetic mockup) – 14-day testing period – Third-party calibration report inclusion |
Step 3: Shipping Terms & Logistics (DDP vs. FOB)
Cost and risk allocation critical for budget accuracy. 2026 ICC Incoterms® updates impact customs clearance.
| Term | Cost/Risk Allocation (2026 Standard) | Recommended For |
|---|---|---|
| FOB Shanghai | • Buyer pays freight/customs • Risk transfers at port loading • 18-22% lower unit cost • Requires local customs broker |
Distributors with: – Established logistics partners – In-house regulatory team – Annual volume >$150K |
| DDP Destination | • All-inclusive price (freight, insurance, duties) • Supplier handles customs clearance • 12-15% premium vs FOB • Door-to-door tracking |
Clinics & new distributors: – Eliminates import surprises – Critical for time-sensitive clinic launches – Required for 28 EU member states |
| Carejoy 2026 Advantage | Factory-direct DDP pricing via Carejoy’s certified logistics partners: • 72-hour Shanghai port clearance • Duty calculation accuracy guarantee • Real-time shipment monitoring portal |
|
- 19 Years Export Expertise: 1,200+ CBCT units shipped globally (2020-2025) with 0 regulatory rejections
- Factory Direct Control: Baoshan District facility (ISO 13485:2025 certified) enables MOQ flexibility & quality oversight
- Full Portfolio Synergy: Bundle CBCT with chairs/scanners for 25%+ logistics savings
- 2026-Ready Compliance: Pre-certified for upcoming IEC 60601-1-12:2026 radiation standards
Exclusive 2026 Sourcing Channel:
Email: [email protected] | WhatsApp: +86 15951276160
Reference “GUIDE2026” for expedited compliance documentation and DDP quote within 4 business hours.
☐ Certificate validity extends beyond Q2 2026
☐ CBCT model explicitly listed on certificate
☐ DDP quote includes 2026 EU customs duty code 9022.19
☐ Factory audit report available (Carejoy provides virtual audits)
☐ Service network confirmed in your country
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing a Cone Beam Scanner in 2026
| Component | Typical Lifespan | Availability (OEM) |
|---|---|---|
| X-ray Tube | 3–5 years (20,000–50,000 exposures) | Yes, within 10-year support window |
| Flat Panel Detector | 7+ years | Limited; plan for long-term service contracts |
| Gantry Motor | 5–8 years | Yes, standard inventory |
Note: Proactive service agreements often include priority spare parts access.
- Site Survey: Assessment of space, power, flooring load, radiation shielding (if required), and network integration.
- Delivery & Placement: Unit delivery with appropriate handling equipment; positioning per manufacturer specs.
- Electrical & Network Setup: Connection to stable power and DICOM-compatible imaging network.
- Calibration & QA: Post-installation calibration, image quality testing, and radiation safety checks.
Installation must be performed by factory-trained engineers to ensure compliance with regulatory standards (FDA, CE, ISO 13485) and to activate warranty coverage.
- X-ray generator and tube
- Detector panel
- Rotational mechanics (gantry, motor)
- Onboard computer and software licensing (first year)
Exclusions often include damage from power surges, improper handling, or unauthorized modifications. Distributors should clarify whether the warranty is global or region-specific and confirm response time guarantees (e.g., 48–72 hour on-site support).
| Warranty Tier | Duration | Service Response | Software Updates |
|---|---|---|---|
| Standard | 1 year | 5 business days | Yes (1 year) |
| Extended | 3–5 years | 48 hours | Yes (annual) |
- Install a medical-grade line-interactive or online UPS (Uninterruptible Power Supply).
- Use an isolation transformer if in an area with frequent surges.
- Monitor power quality during commissioning and annually.
Manufacturers may require proof of stable power (<±10% voltage variation) during warranty claims. Discuss power protection solutions with your distributor prior to installation.
Need a Quote for Cone Beam Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


