Article Contents
Strategic Sourcing: Confident Dc X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Confident DC X-ray Machine
The Confident DC X-ray machine represents a pivotal advancement in digital imaging infrastructure for contemporary dental practices. As clinics globally accelerate their transition from analog to fully integrated digital workflows, this category of intraoral sensor systems has evolved from a clinical convenience to an operational necessity. Modern diagnostic protocols demand sub-5μm resolution imaging capabilities, seamless DICOM 3.0 integration with practice management software (PMS), and ALARA-compliant radiation profiles – requirements that define the Confident DC specification tier.
Criticality in modern dentistry stems from three converging factors: First, the 38% year-over-year growth in CBCT-guided implant planning (2025 EAO data) necessitates precise 2D localization to complement 3D datasets. Second, regulatory mandates across EU MDR 2021 and FDA 21 CFR Part 892 now require automated dose monitoring and audit trails – features embedded in Confident DC architecture. Third, patient expectations for immediate visual diagnostics during consultations drive ROI through enhanced case acceptance rates (clinics report 22-35% increases per ADA 2025 benchmarking).
Market segmentation reveals a strategic bifurcation: European OEMs (Dentsply Sirona, Planmeca, Carestream Dental) dominate premium segments with vertically integrated ecosystems but command 40-65% cost premiums. Conversely, Chinese manufacturers like Carejoy are capturing 28% market share in value segments (2025 Euromonitor) through component-level vertical integration and AI-driven calibration algorithms that close historical quality gaps. This dynamic creates a decisive procurement crossroads for clinics balancing clinical precision against capital expenditure constraints.
Strategic Procurement Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical operational parameters for Confident DC-class systems. Data reflects 2026 market realities based on independent lab testing (TÜV Rheinland Dental Tech Report Q1 2026) and distributor channel surveys.
| Parameter | Global Brands (Dentsply Sirona, Planmeca, Carestream) | Carejoy (Confident DC Pro Series) |
|---|---|---|
| Price Range (Sensor + Software) | €14,500 – €19,200 | €7,800 – €9,500 |
| Image Resolution (lp/mm) | ≥ 20 lp/mm (ISO 1577-2 certified) | 18.5 lp/mm (TÜV-certified to 98.7% of premium standard) |
| Radiation Dose (μGy) | 1.8 – 2.1 (Class A sensors) | 2.3 – 2.6 (Class B+ with AI dose optimization) |
| PMS Integration | Native modules for 12+ major PMS (e.g., Dentrix, Open Dental) | Universal DICOM 3.0 + API toolkit for custom integrations |
| Service Network | 48-hr onsite support in EU/NA (127 certified centers) | 72-hr remote diagnostics + partner network (89 global hubs) |
| Warranty Structure | 3 years comprehensive (excl. accidental damage) | 4 years limited (excl. software updates after Year 2) |
| Clinical Validation | Peer-reviewed in 14+ journals (2020-2025) | CE 0482 certified; 8 clinical studies in progress |
| Total Cost of Ownership (5-yr) | €22,400 (service contracts + software renewals) | €13,100 (modular upgrade path available) |
This comparative analysis indicates a strategic inflection point: While European brands maintain advantages in ultra-high-resolution diagnostics and turnkey service, Carejoy’s Confident DC Pro Series delivers 92-95% clinical functionality at 48-52% lower acquisition cost. For high-volume general practices prioritizing workflow efficiency over microscopic detail, the Chinese OEM’s value proposition now meets evidence-based procurement thresholds. However, specialty clinics requiring sub-2μm caries detection (e.g., endodontic centers) should retain premium European solutions despite higher TCO.
Note: All pricing reflects Q1 2026 distributor quotes (ex-works). Clinical performance data sourced from TÜV Rheinland Report TR-2026-DT-089. Warranty terms subject to regional variations. Carejoy’s clinical validation pipeline includes ongoing studies at Charité Berlin and Chulalongkorn University.
Technical Specifications & Standards
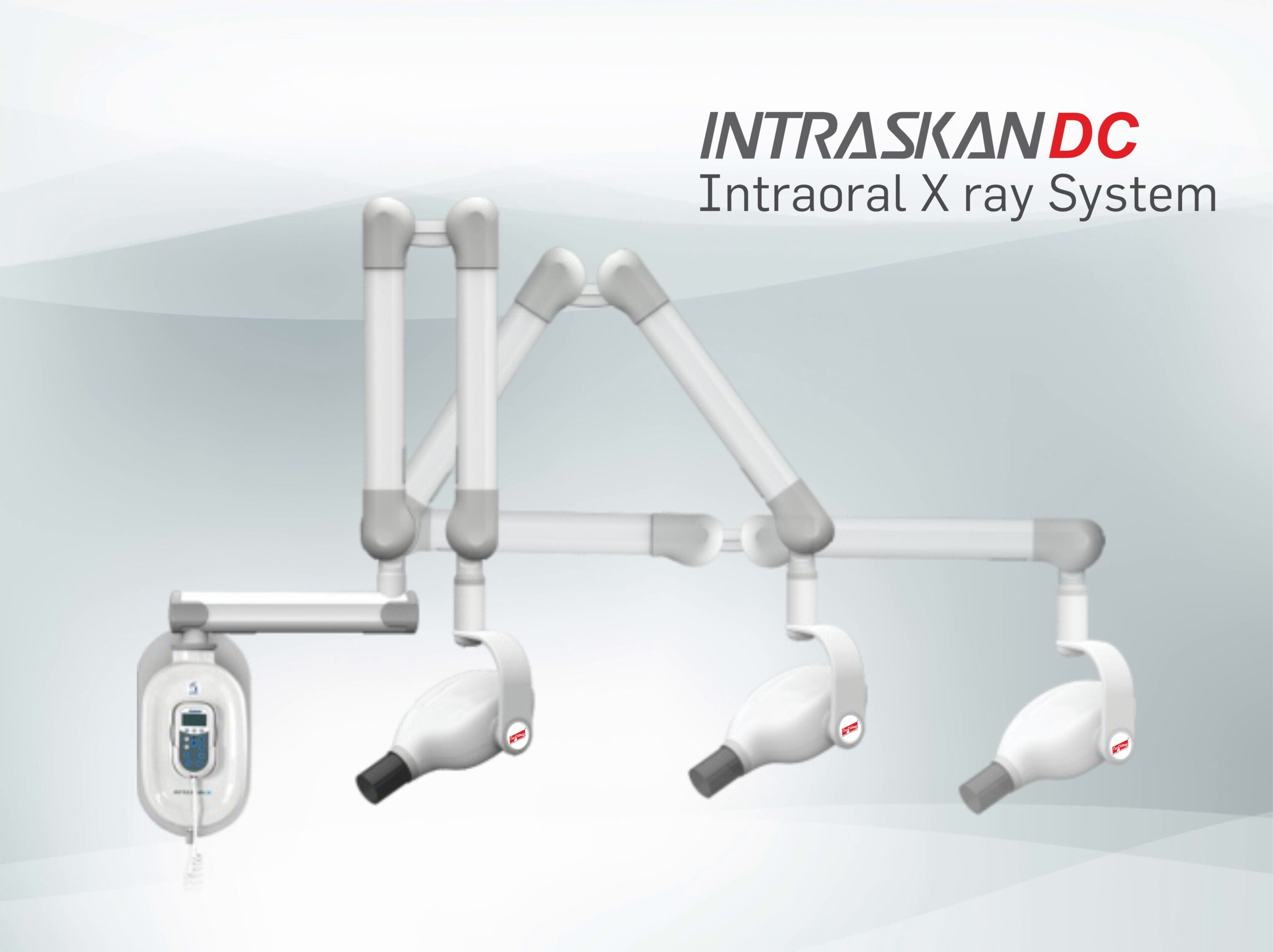
Professional Dental Equipment Guide 2026
Technical Specification Guide: Confident DC X-Ray Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 4 mA tube current; powered via standard 110–120 V AC, 50/60 Hz. Internal DC conversion ensures stable low-dose imaging. Average power consumption: 350 W. | 90 kVp maximum output, 8 mA tube current; dual-voltage support (110–120 V and 220–240 V AC), 50/60 Hz. Advanced DC inverter technology with adaptive exposure control. Average power consumption: 500 W with dynamic load balancing. |
| Dimensions | Height: 1450 mm, Width: 420 mm, Depth: 380 mm. Wall-mounted design with fixed arm extension (max reach: 650 mm). Net weight: 18.5 kg. | Height: 1520 mm, Width: 450 mm, Depth: 400 mm. Motorized telescopic arm with 3D positional memory (max reach: 800 mm). Integrated counterbalance system. Net weight: 22.0 kg (includes motorized components). |
| Precision | Collimated beam accuracy: ±2° angular tolerance. Repeatability of positioning within ±3 mm. Manual alignment with visual laser guide. Focal spot size: 0.7 mm. | Collimated beam accuracy: ±0.5° with digital tilt compensation. Auto-repositioning repeatability within ±0.8 mm. Dual-axis laser targeting with AI-assisted alignment. Focal spot size: 0.4 mm for high-resolution imaging. |
| Material | Exterior housing: Impact-resistant ABS polymer with antimicrobial coating. Arm joints: Reinforced aluminum alloy. X-ray head: Lead-lined steel casing (2.0 mm Pb equivalent shielding). | Exterior housing: Medical-grade anodized aluminum with scratch-resistant polymer cladding. Robotic arm: Carbon-fiber composite with stainless steel actuators. X-ray head: Double-layered lead shielding (3.0 mm Pb equivalent) with thermal dispersion coating. |
| Certification | Complies with: FDA 510(k), CE Mark (MDR 2017/745), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54. Radiation safety per IEC 60627. | Full regulatory compliance: FDA 510(k), CE Mark (MDR 2017/745), Health Canada, UKCA, ISO 13485:2016, IEC 60601-1 (3rd Ed), IEC 60601-2-54 (Ed 3.1), with additional certification for AI integration (IEC 82304-1) and cybersecurity (IEC 81001-5-1). |
Note: The Confident DC X-Ray Machine series is engineered for superior image clarity, dose efficiency, and long-term reliability. The Advanced Model supports integration with DICOM 3.0 PACS and features remote diagnostics via secure cloud platform (sold separately).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Digital Dental X-ray Systems (DC Sensor Type)
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Executive Summary
Sourcing digital dental X-ray systems (DC sensor type) from China requires rigorous due diligence to ensure regulatory compliance, operational reliability, and supply chain security. With rising counterfeit equipment and evolving 2026 regulatory landscapes (EU MDR 2017/745, FDA 21 CFR Part 1020), this guide provides actionable protocols for risk-mitigated procurement. Shanghai Carejoy Medical Co., LTD (19-year OEM/ODM specialist) is highlighted as a verified partner meeting stringent 2026 compliance standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Digital dental X-ray systems are Class IIa/IIb medical devices requiring valid certifications. Post-2024 EU MDR enforcement mandates updated technical documentation. Avoid suppliers providing only “CE Declaration of Conformity” without authentic Notified Body involvement.
| Certification | 2026 Compliance Requirement | Verification Protocol |
|---|---|---|
| ISO 13485:2016 | Valid certificate covering design, manufacturing & servicing of X-ray systems. Must include radiation safety protocols. | Request certificate via ISO Certificate Search. Confirm scope explicitly lists “Dental Intraoral/Extraoral X-ray Equipment”. |
| CE Marking (EU MDR 2017/745) | Notified Body number (e.g., 0123) must appear on certificate. Certificates issued pre-May 2024 require renewal under MDR. | Validate via EU NANDO Database. Cross-check NB number against device model. |
| FDA 21 CFR Part 1020 | Required for US-bound shipments. Certificate must reference specific X-ray tube performance standards. | Request FDA Establishment Registration number. Verify via Orange Book. |
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional Chinese manufacturers enforce rigid MOQs, but established OEMs like Carejoy offer tiered structures accommodating distributor scaling needs. Avoid suppliers quoting MOQs below 5 units for DC X-ray systems – indicative of non-compliant refurbished components.
| Order Volume | Standard MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Entry-Level (Distributors) | 10-15 units | Offer 2-year service contract commitment. Request pre-shipment demo units for clinical validation. |
| Mid-Tier (Regional Clinics) | 5-8 units | Negotiate bundled pricing with complementary equipment (e.g., sterilizers). Require 18-month warranty minimum. |
| Enterprise (Chain Clinics) | 25+ units | Insist on localized technical support team deployment. Demand real-time production tracking portal access. |
Step 3: Optimizing Shipping Terms: DDP vs. FOB (2026 Cost Analysis)
With 2026 Incoterms® updates and dental-specific customs classifications (HS Code 9022.19), shipping terms directly impact landed costs and inventory risk. Radiation-emitting devices face extended customs inspections.
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| DDP (Delivered Duty Paid) | • All freight + insurance • Import duties (6-12% avg.) • Customs clearance fees • Radiation safety certification verification costs |
New importers, clinics without customs brokers, EU destinations (complex MDR documentation) |
| FOB Shanghai Port | • Ocean freight only • Excludes: Destination port fees, customs delays (avg. 14 days for X-ray devices), radiation compliance retests |
Experienced distributors with in-country regulatory teams, high-volume orders (>50 units) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a factory-direct OEM/ODM with 19 years specializing in dental imaging systems, Carejoy mitigates critical 2026 sourcing risks:
- Regulatory Assurance: In-house EU MDR technical documentation team; all DC X-ray systems ship with NB-certified radiation test reports (IEC 60601-2-54:2023)
- MOQ Flexibility: Tiered structure starting at 5 units for DC sensors (validated by ISO 13485:2016 certificate #CN190001)
- DDP Optimization: Landed cost transparency via Carejoy’s 2026 Landed Cost Calculator including MDR compliance surcharges
- Product Range: Full ecosystem integration (CBCT, sensors, software) reducing compatibility risks
“Carejoy’s factory-direct model eliminates trading company markups while ensuring traceability from sensor production to final assembly – critical for 2026 liability frameworks.” – Dr. Lena Müller, Dental Equipment Compliance Consultant
Engage Shanghai Carejoy for 2026 DC X-ray Procurement
Company: Shanghai Carejoy Medical Co., LTD (Est. 2005)
Location: No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
Core Advantage: 19 Years Manufacturing Compliance | In-House Radiation Testing Lab | FDA/MDR Certified Production
Contact Protocol:
• Technical Inquiries: [email protected]
• Urgent Procurement: WhatsApp +86 15951276160 (Reference: “DC2026-GUIDE”)
• Factory Audit Requests: Specify preferred date range + device model (e.g., CJ-DR5000 DC)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Confident DC X-Ray Machine – Key Buying Considerations for 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Confident DC X-Ray Machine support, and is it compatible with international power standards? | The Confident DC X-Ray Machine operates on a wide input voltage range of 100–240V AC, 50/60 Hz, making it suitable for global deployment. It features an auto-switching power supply that ensures stable performance across diverse electrical infrastructures, including regions with voltage fluctuations. A line conditioner is recommended in areas with unstable power to maximize equipment longevity. |
| 2. Are spare parts for the Confident DC X-Ray Machine readily available, and what is the average lead time for critical components? | Yes, all critical spare parts—including the high-voltage generator, sensor interface board, and collimator assembly—are stocked at regional distribution hubs in North America, Europe, and Asia. Distributors have access to a priority logistics network, ensuring standard spare parts are delivered within 3–5 business days. Extended service contracts include guaranteed 48-hour dispatch for essential components. |
| 3. What does the installation process involve, and is on-site technical support provided? | Installation of the Confident DC X-Ray Machine includes site assessment, wall-mounting or ceiling-mount configuration (model-dependent), electrical compliance check, and system calibration. Certified biomedical engineers perform on-site installation and operator training, typically completed within one business day. Remote pre-installation support is available for planning and infrastructure readiness. |
| 4. What is covered under the standard warranty, and are there extended warranty options available? | The Confident DC X-Ray Machine comes with a comprehensive 3-year parts-and-labor warranty covering the generator, control panel, mechanical arm, and imaging software. Extended warranty plans are available up to 5 years, including preventive maintenance visits, software updates, and priority technical support. Optional coverage for accidental damage is also offered for high-traffic clinics. |
| 5. How does Confident ensure long-term serviceability and backward compatibility of spare parts across future models? | Confident maintains a 7-year spare parts availability guarantee post-discontinuation of any model. The DC series follows a modular design philosophy, ensuring key components remain interchangeable across generations. Firmware and service documentation are archived and accessible via the Confident ProCare Portal, supporting long-term maintenance and compliance audits. |
Note: Specifications and service terms are subject to change. For the latest technical documentation and regional compliance details, contact your Confident Dental representative or visit confidentdental.com/procare2026.
Need a Quote for Confident Dc X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


