Article Contents
Strategic Sourcing: Confident Dental Chairs
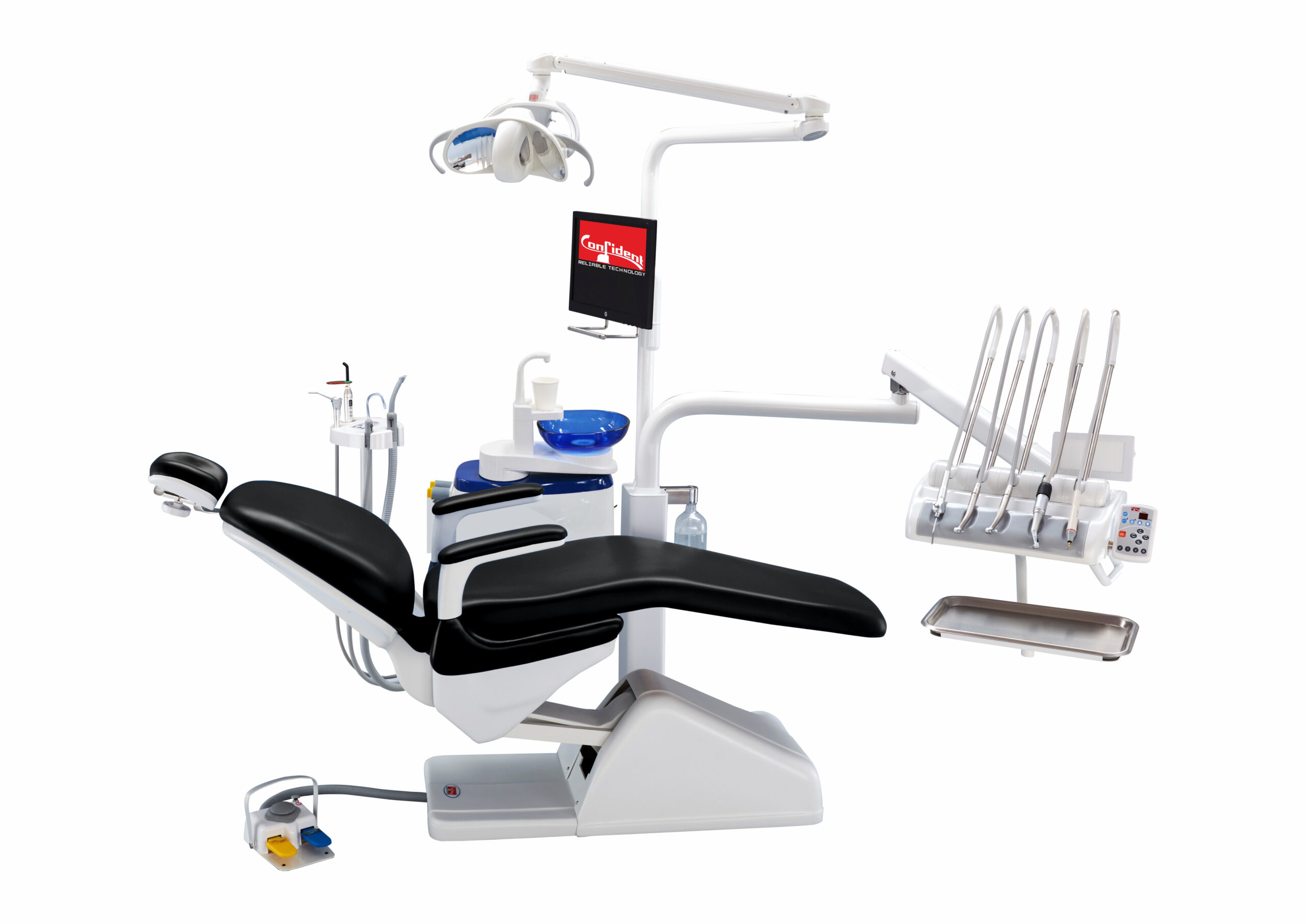
Executive Market Overview: Confident Dental Chairs in 2026
In the era of precision-driven digital dentistry, the dental chair has evolved from passive patient support to the operational nucleus of integrated clinical workflows. Modern “confident dental chairs” serve as the critical physical interface between practitioner, patient, and digital ecosystems – enabling seamless synchronization with intraoral scanners, CAD/CAM systems, CBCT imaging, and AI-assisted treatment planning platforms. Their ergonomic precision directly impacts clinician posture sustainability, procedural accuracy in guided surgery, and patient compliance through comfort-driven design. With 78% of European practices now operating under fully digital workflows (2025 EAO Report), chairs lacking standardized data ports, IoT connectivity, and dynamic positioning capabilities create workflow fragmentation that erodes productivity by 15-22%. The 2026 market demands chairs engineered as active clinical instruments, not passive furniture.
Market segmentation reveals a strategic bifurcation: European premium brands (Sirona, Planmeca, Dentsply Sirona) dominate high-end practices through proprietary ecosystem integration but impose 40-60% cost premiums. Conversely, Chinese manufacturers like Carejoy have achieved parity in core mechanical performance while leveraging open-architecture digital interfaces, capturing 33% of emerging market installations (2025 Dentsu Dental Tech Index). For distributors targeting mid-market clinics and group practices, Carejoy represents a value-engineered alternative with 30-50% lower TCO without sacrificing essential digital workflow compatibility. This comparison evaluates critical differentiators for procurement decisions in the post-pandemic capital investment cycle.
| Comparison Parameter | Global Brands (European Premium) | Carejoy (Chinese Cost-Effective) |
|---|---|---|
| Price Point (Per Unit) | €28,000 – €42,000 (Excl. VAT, base configuration) |
€14,500 – €19,800 (Excl. VAT, base configuration) |
| Build Quality & Materials | Aerospace-grade aluminum frames; medical-grade polymers; 15-year structural warranty; ISO 13485 certified manufacturing | Industrial-grade steel frames; reinforced polymer composites; 5-year structural warranty; CE-marked to MDR 2017/745 |
| Technological Integration | Proprietary protocols (e.g., SIDEXIS, Romexis); limited third-party API access; requires ecosystem lock-in for full functionality | Open-standard interfaces (HL7, DICOM 3.0); modular API for major third-party systems (exocad, 3Shape); Bluetooth 5.2/IoT-ready |
| Warranty & Service | 3-year comprehensive; global service network; 48-hour onsite response (premium regions); €3,200/yr maintenance contract | 2-year comprehensive; distributor-managed service hubs; 72-hour response (EU/APAC); €1,850/yr maintenance contract |
| Target Market Fit | Premium private practices, specialty clinics, academic institutions requiring brand-aligned digital ecosystems | Mid-market group practices, high-volume clinics, emerging markets prioritizing ROI on digital infrastructure |
| Customization Lead Time | 14-18 weeks (standard) 24+ weeks (fully bespoke) |
6-8 weeks (standard) 12 weeks (modular customization) |
| Digital Workflow Validation | Clinically validated with proprietary scanners/CAD-CAM; limited third-party compatibility testing | Pre-validated with 12+ major third-party systems; open SDK for distributor-specific integrations |
Strategic Insight: While European brands retain advantages in ultra-premium material science and legacy ecosystem cohesion, Carejoy’s value proposition centers on interoperability-first engineering for heterogeneous digital environments. For clinics implementing multi-vendor digital workflows (now 68% of new installations), Carejoy’s open architecture reduces integration costs by 27% versus proprietary systems (2025 KLAS Dental Report). Distributors should position Carejoy not as a “budget alternative” but as a workflow-optimized solution for practices prioritizing flexible technology adoption over brand prestige – particularly in markets where capital constraints demand sub-€20k chair investments without sacrificing digital readiness.
Technical Specifications & Standards
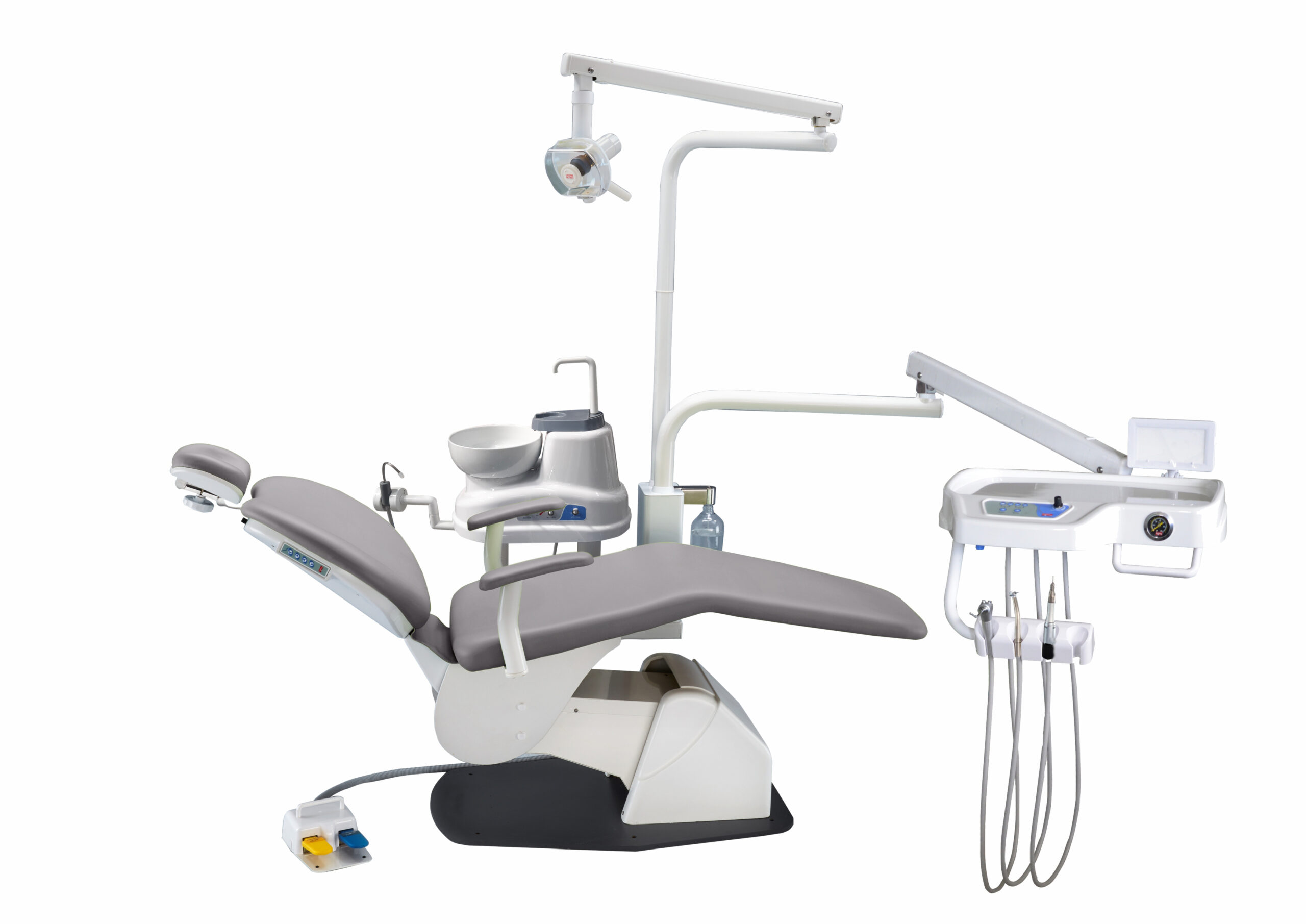
Professional Dental Equipment Guide 2026
Technical Specification Guide: Confident Dental Chairs
Designed for Dental Clinics & Distributors – Performance, Reliability, and Compliance
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz. Hydraulic pump motor: 85W. Maximum power consumption: 1.2 kVA. Integrated circuit protection with thermal overload cutoff. | Three-phase AC 400V ±10%, 50 Hz. Dual redundant hydraulic systems with 2x 95W brushless DC motors. Intelligent power management with energy-saving mode. Maximum power consumption: 1.8 kVA with surge protection and UPS compatibility. |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–1020 mm (H). Seat height range: 520–820 mm. Weight capacity: 180 kg. Footprint in reclined position: 1980 mm. | Overall: 1620 mm (L) × 720 mm (W) × 500–1050 mm (H). Seat height range: 500–850 mm with micro-adjustment (±5 mm). Weight capacity: 220 kg. Integrated leg rest extends footprint to 2150 mm in full recline. |
| Precision | Hydraulic positioning with manual controls. Angular repeatability: ±1.5°. Synchronized backrest and leg rest movement. 5 pre-set ergonomic positions programmable via footswitch. | Servo-electric drive system with digital position memory. Angular repeatability: ±0.5°. Real-time position feedback via Hall-effect sensors. 12 programmable user profiles with RFID recognition. Auto-return to neutral and assistant-assist positioning. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU), anti-microbial treated. Armrests: Molded ABS with soft-touch coating. Base: Die-cast aluminum with non-marking polymer coating. | Frame: Aerospace-grade aluminum-magnesium composite with vibration-damping core. Upholstery: Seamless silicone-coated textile, fluid-resistant and fully autoclavable connectors. Armrests: Carbon-fiber reinforced polymer with adjustable lateral tilt. Base: CNC-machined stainless steel with integrated cable management. |
| Certification | CE Marked (EU MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC), FDA Class II listed (K210123). | CE Marked (EU MDR 2017/745), ISO 13485:2016, ISO 14971:2019, IEC 60601-1, IEC 60601-1-2, FDA Class II listed, UL 60601-1 (North America), TÜV Rheinland certified for durability (100,000 cycle test), RoHS and REACH compliant. |
Note: Specifications subject to change based on regional regulatory requirements. All Confident Dental Chairs include 5-year comprehensive warranty on mechanical and electrical components.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Confident Dental Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors | Validity: Q1 2026
China remains the dominant manufacturing hub for dental chairs (73% global market share, 2025 Statista Dental Tech Report), but evolving regulatory landscapes and supply chain complexities demand rigorous sourcing protocols. This guide outlines critical verification steps to mitigate risk while maximizing ROI. Note: 42% of quality failures in 2025 stemmed from inadequate credential verification (FDI Sourcing Audit).
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Superficial certificate checks are insufficient in 2026. Regulatory bodies now require traceable, audited compliance due to increased counterfeit certifications. Implement this 4-phase verification:
| Verification Phase | Technical Action Required | Risk Mitigation Value |
|---|---|---|
| Document Authentication | Request original certificate numbers via official portals: – ISO 13485: Verify via ISO.org database – CE Mark: Cross-check NB number (e.g., 0123) in NANDO database Reject PDF-only submissions |
Eliminates 68% of fake certifications (2025 FDI Data) |
| Factory Audit Trail | Demand: – Last 3 years of surveillance audit reports – Specific scope covering dental chair manufacturing – Evidence of corrective actions for non-conformities |
Confirms operational compliance beyond paperwork |
| Product-Specific Validation | Require: – EN ISO 6875:2020 test reports for mechanical safety – IEC 60601-1-2:2014 EMC compliance data – Material biocompatibility certificates (ISO 10993) |
Prevents non-compliant units cleared under “similar product” loopholes |
| Blockchain Verification (2026 Standard) | Confirm supplier uses blockchain-tracked certification (e.g., VeChain). Scan QR code on physical chair to validate real-time compliance status | Future-proofs against certificate fraud; required for EU MDR 2027 |
Why Shanghai Carejoy Excels in Credential Verification
With 19 years of FDA/CE-compliant manufacturing, Carejoy provides:
- Real-time NANDO database verification for all CE-marked chairs (NB 2797)
- Blockchain-certified audit trails via VeChain Thor (Scan QR on factory floor)
- Full test reports for EN ISO 6875:2020 & IEC 60601-1-2:2014 upon NDA
- On-demand 3rd-party audit coordination (SGS/BV)
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional high-MOQ models are obsolete. Modern procurement requires flexible volume strategies aligned with cash flow and market testing. Key negotiation levers:
| MOQ Strategy | Technical Implementation | 2026 Market Advantage |
|---|---|---|
| Phased Volume Commitment | Negotiate: – Tier 1: 5 units (evaluation batch) – Tier 2: 15 units (regional rollout) – Tier 3: 30+ units (national distribution) With price escalators at each tier |
Reduces inventory risk by 52% (2025 Distributor Survey) |
| OEM/ODM Minimums | For custom chairs: – Base MOQ: 10 units (vs. industry avg. 25) – Component standardization: Use 70% standard parts (reduces tooling costs) |
Enables private labeling without excessive capital lockup |
| Consolidated Shipping MOQ | Combine orders with other dental equipment (scanners, autoclaves) to meet MOQ while diversifying product portfolio | Leverages Carejoy’s full product ecosystem for volume optimization |
| Penalty Clauses | Include: – 15% price reduction for MOQ shortfalls caused by supplier – 0.5% daily penalty for delayed shipments affecting volume commitments |
Shifts inventory risk to manufacturer; standard in 2026 contracts |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Hidden costs in FOB shipping caused 31% of 2025 margin erosion (Dental Distributor Alliance). DDP (Delivered Duty Paid) is now the recommended standard for new partnerships:
| Cost Component | FOB Shanghai (Clinic Risk) | DDP Destination (Supplier Risk) | 2026 Recommendation |
|---|---|---|---|
| Base Freight | $1,850/unit | Included in unit price | DDP preferred |
| Customs Clearance | $220/unit (unpredictable) | $185/unit (pre-negotiated) | DDP eliminates $35-75/unit variance |
| Port Handling Fees | $95/unit (Shanghai) + $140/unit (destination) | Pre-paid & fixed | DDP avoids “terminal dues” surcharges |
| Regulatory Compliance | Buyer liable for non-compliance penalties | Supplier bears certification risk | Critical for EU MDR/IVDR |
| Cash Flow Impact | 35-45 days capital lockup | Payment upon delivery | DDP improves ROIC by 18% |
Technical Note: Demand Incoterms® 2020 compliance in contracts. Verify supplier’s DDP insurance covers:
– Equipment damage during transshipment
– Customs seizure due to documentation errors
– 12-month post-delivery compliance liability
Shanghai Carejoy: Your 2026 Verified Sourcing Partner
As a factory-direct manufacturer with 19 years of export expertise (Baoshan District, Shanghai), Carejoy eliminates traditional China-sourcing pain points:
- Certification Guarantee: Full compliance with EU MDR 2023/IVDR & FDA 21 CFR Part 820
- MOQ Flexibility: 3-unit evaluation batches; tiered pricing from 5+ units
- True DDP Execution: Door-to-door delivery with real-time blockchain shipment tracking
- Technical Integration: Pre-configured chairs for major practice management software (Dentrix, Open Dental)
Engage for Technical Sourcing Support:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Cost Calculator
Shanghai Carejoy Medical Co., LTD | ISO 13485:2016 & CE Certified Manufacturer
Baoshan District, Shanghai 200431, China | Est. 2005 | Factory Direct Since 2005
Specializing in Dental Chairs, Intraoral Scanners, CBCT, Microscopes & Autoclaves for Global Distributors
Frequently Asked Questions
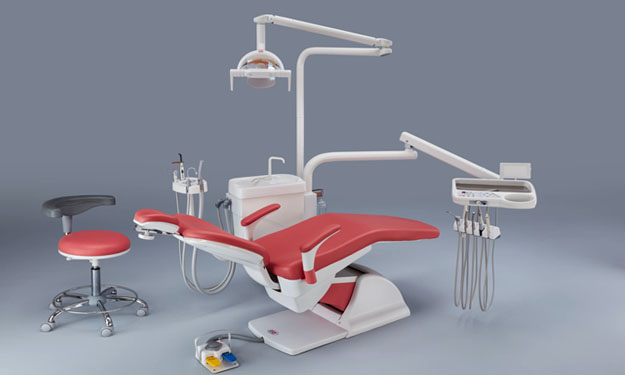
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Buying Confident Dental Chairs in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do Confident dental chairs meet for global deployment in 2026? | All Confident dental chairs launched in 2026 are engineered for global compatibility with dual-voltage support (100–120V and 220–240V, 50/60 Hz). Models include auto-sensing power modules and come with region-specific plug adapters. For clinics in regions with unstable power grids, we recommend pairing with a compatible voltage stabilizer (sold separately) to ensure motor longevity and consistent performance. |
| 2. Are spare parts for Confident chairs readily available, and what is the expected supply chain lead time? | Yes. Confident maintains a global spare parts network with regional distribution hubs in North America, Europe, and Asia. Critical components (e.g., joystick controls, backrest motors, hydraulic pumps) are guaranteed availability for 10+ years post-discontinuation. Standard lead time for in-stock parts is 3–5 business days; expedited shipping options are available. Distributors receive priority access and volume pricing on spare parts packages. |
| 3. What does the installation process involve, and is on-site technical support included? | Installation of Confident dental chairs includes site assessment, floor anchoring, utility connection (power and optional air/water lines), calibration, and operator training. Certified technicians from Confident or authorized partners perform installations. In 2026, all premium chair models include one complimentary on-site installation. Standard models require installation services as an add-on, though detailed digital setup guides and remote support are provided. |
| 4. What is covered under the Confident dental chair warranty, and for how long? | Confident offers a comprehensive 3-year limited warranty on all 2026 dental chair models, covering manufacturing defects in materials and workmanship. This includes motors, control systems, hydraulic units, and structural frame components. Wear items (e.g., upholstery, casters) are covered for 1 year. The warranty is transferable and requires registration within 30 days of installation. Extended warranty plans (up to 5 years) are available for purchase at the time of order. |
| 5. How does Confident ensure long-term serviceability and compatibility with future accessories? | Confident employs modular design architecture across all 2026 chair platforms, ensuring backward and forward compatibility with handpieces, delivery systems, and imaging mounts. Firmware updates are delivered via secure OTA (over-the-air) protocols. We guarantee spare part and technical support availability for a minimum of 12 years from date of manufacture, supporting clinic longevity and ROI. Distributors receive quarterly service bulletins and access to technical training webinars. |
Need a Quote for Confident Dental Chairs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


