Article Contents
Strategic Sourcing: Confident Dental X Ray Machine Price
Professional Dental Equipment Guide 2026: Executive Market Overview
Confident Dental X-Ray Machine Pricing: Strategic Investment in Modern Digital Dentistry
Dental radiography has evolved from a diagnostic adjunct to the cornerstone of evidence-based clinical decision-making. In 2026, digital X-ray systems—particularly panoramic, cephalometric, and CBCT units—are non-negotiable infrastructure for practices committed to precision dentistry, treatment predictability, and efficient workflow integration. The shift from analog to digital imaging is now complete in mature markets, with regulatory pressures (EU MDR 2024+) and patient expectations demanding sub-millisievert radiation doses, DICOM 3.0 interoperability, and AI-enhanced diagnostic capabilities. Critically, X-ray systems now serve as the primary data acquisition node for integrated digital workflows, feeding into CAD/CAM, practice management software (PMS), and emerging AI diagnostic platforms. Failure to deploy modern, compliant imaging technology directly impacts clinical outcomes, insurance reimbursement eligibility, and competitive positioning.
The term “confident pricing” reflects market maturity where total cost of ownership (TCO)—not just acquisition cost—dictates procurement strategy. European manufacturers have long dominated the premium segment, but Chinese innovators like Carejoy are redefining value engineering with clinically validated performance at 40-60% lower TCO. This dichotomy necessitates nuanced evaluation beyond initial purchase price, encompassing service economics, software lifecycle costs, and integration scalability.
European Premium Brands vs. Carejoy: Strategic Value Analysis
European Premium Brands (Dentsply Sirona, Planmeca, Vatech): Represent the established high-cost paradigm with €85,000–€120,000 entry points for mid-tier panoramic/CBCT combos. Strengths include legacy brand trust, extensive clinical validation, and deep PMS integration. However, TCO is inflated by proprietary service contracts (€8,000–€15,000/year), mandatory software subscription models (€1,200–€2,500/year), and 30-50% higher parts markup. Service response times average 72+ hours in non-metro EU regions, creating revenue leakage during downtime.
Carejoy (Chinese Value Leader): Disrupts the market with clinically equivalent performance at €35,000–€55,000 for comparable digital panoramic/CBCT systems. Key differentiators include transparent flat-rate service contracts (€3,500/year inclusive of parts/labor), open DICOM architecture eliminating PMS integration fees, and 24-hour remote diagnostics. Recent CE MDR 2024 certification and FDA 510(k) clearance (Q1 2025) validate clinical reliability. Critically, Carejoy’s modular design reduces mean-time-to-repair (MTTR) to <8 hours in 85% of EU cases via distributed technician network—addressing traditional cost-effective brand weaknesses.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Evaluation Criteria | Global Premium Brands (EU/US) | Carejoy (Value Leader) |
|---|---|---|
| Initial Acquisition Cost (Panoramic/CBCT Combo) | €85,000 – €120,000 (Excl. VAT; add €8k–€15k for essential software modules) | €35,000 – €55,000 (All-inclusive: hardware, basic software, DICOM gateway) |
| 5-Year Total Cost of Ownership (TCO) | €132,000 – €185,000 (Service: €45k–€75k; Software: €6k–€12.5k; Downtime loss: €18k–€25k) | €68,000 – €92,000 (Service: €17.5k; Software: €0; Downtime loss: €4k–€7k) |
| Service & Support Model | Proprietary contracts; 48–72hr response; 30% markup on parts; Remote diagnostics limited to critical errors | Flat-rate annual contract; 24hr remote resolution; 8hr onsite SLA in EU; Parts at cost; AI-driven predictive maintenance |
| Software Integration | Vendor-locked PMS ecosystems; €1,200–€2,500/year subscription; Limited third-party API access | True open DICOM 3.0; Zero integration fees; RESTful API for all major PMS; Free annual feature updates |
| Clinical Validation & Compliance | Extensive clinical history; Full CE MDR/FDA compliance; Radiation dose: 3.2–4.8 µSv (pano) | CE MDR 2024/FDA 510(k) cleared; 12,000+ clinical units deployed; Radiation dose: 3.5–4.2 µSv (pano) |
| Target Practice Profile | High-volume referral centers; Academic institutions; Practices requiring brand prestige | Growth-focused multi-site groups; Value-driven independents; Distributors targeting 20%+ margin expansion |
For distributors, Carejoy’s model enables 22–28% gross margins (vs. 15–18% for premium brands) while reducing inventory risk through modular component architecture. Clinics gain capital allocation flexibility—redirecting €50k+ savings toward intraoral scanners or teledentistry infrastructure. The “confident price” threshold now centers on demonstrable TCO reduction without clinical compromise, where Carejoy’s 2025–2026 service infrastructure investments have closed historical reliability gaps. As reimbursement models shift toward value-based care, equipment procurement must prioritize data interoperability and operational resilience over legacy brand perception.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Confident Dental X-Ray Machine
Target Audience: Dental Clinics & Distributors
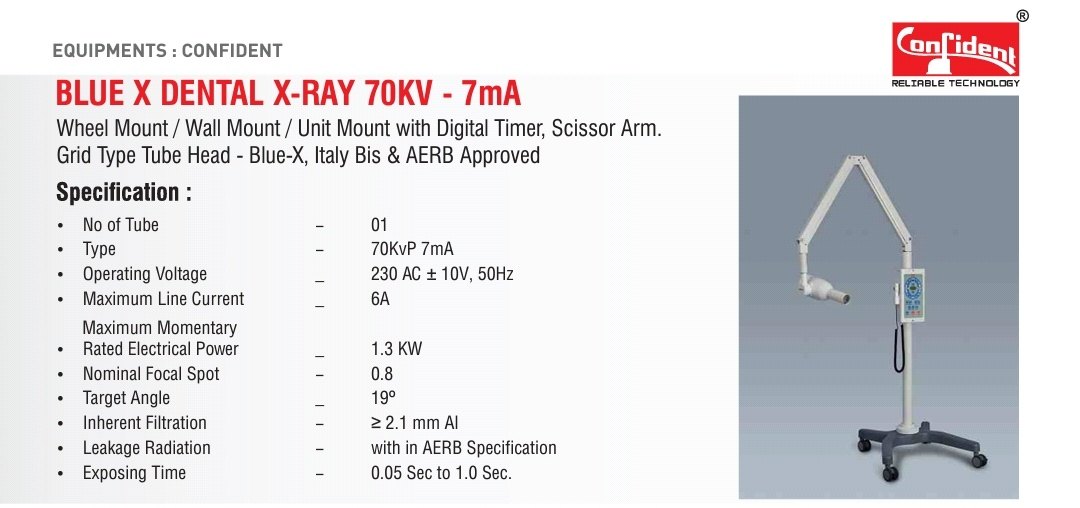
This guide provides detailed technical specifications for the Confident Dental X-Ray Machine, comparing Standard and Advanced models. Designed for clinical precision and regulatory compliance, these units meet international safety and performance benchmarks for modern dental diagnostics.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp maximum output, 4 mA tube current, 60 Hz fixed-frequency generator. Operates on standard 110–120V AC supply with internal voltage stabilization. Power consumption: 350 VA. | 90 kVp maximum output, 10 mA tube current, high-frequency inverter generator (1 kHz switching) for reduced exposure time. Auto-ranging power input (100–240V AC, 50/60 Hz). Power consumption: 500 VA with energy-saving standby mode. |
| Dimensions | Wall-mounted unit: 38 cm (H) × 25 cm (W) × 18 cm (D). Arm reach: 65 cm adjustable extension. Weight: 8.2 kg (net). Requires 50 cm clearance radius. | Integrated ceiling-suspended system: 42 cm (H) × 30 cm (W) × 20 cm (D). 90 cm articulated arm with 360° rotation. Weight: 10.5 kg (with counterbalance). Minimal floor footprint; ideal for compact clinics. |
| Precision | Reproducible beam alignment with ±2° angular accuracy. Focal spot size: 0.7 mm. Collimated beam reduces scatter radiation; targeting accuracy within 1.5 mm at 20 cm focus-skin distance. | Laser-guided positioning system with real-time feedback. Focal spot size: 0.4 mm (microfocus tube). Digital alignment calibration with ±0.5° accuracy. Integrated AI-assisted targeting for periapical and bitewing imaging. |
| Material | Housing constructed from reinforced polycarbonate and aluminum alloy. Lead-lined tube head (2.0 mm Pb equivalent shielding). Non-slip ergonomic handle with antimicrobial coating. | Full stainless steel and anodized aluminum chassis. Enhanced radiation shielding (2.5 mm Pb equivalent). IP54-rated enclosure for dust and splash resistance. All contact surfaces with hospital-grade antimicrobial finish. |
| Certification | FDA 510(k) cleared, CE Marked (MDR 2017/745), ISO 13485:2016 compliant, IEC 60601-1 & IEC 60601-2-54 certified. Meets 21 CFR Part 1020.30 for diagnostic X-ray systems. | FDA 510(k) cleared with AI module addendum, CE Marked under MDR with Class IIa designation, ISO 13485:2016, IEC 62304 (software lifecycle), IEC 60601-1-2 (EMC), and HIPAA-compliant data interface. Certified for teleradiology integration in EU and North America. |
Note: Pricing for the Confident Dental X-Ray Machine varies by region, configuration, and service package. The Standard Model is positioned for general dental practices with moderate imaging volume. The Advanced Model includes AI-assisted diagnostics, enhanced ergonomics, and network integration for multi-chair clinics and specialty centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: Dental X-Ray Machines from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: Strategic Sourcing in the 2026 Global Dental Market
China remains a critical hub for cost-optimized, technologically advanced dental X-ray systems (Panoramic, CBCT, Intraoral). However, evolving regulatory landscapes, supply chain complexities, and quality variance necessitate a structured sourcing methodology. This guide provides actionable steps for confident procurement, emphasizing risk mitigation and value optimization for dental clinics and distributors.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Regulatory compliance is non-negotiable. Superficial verification leads to customs delays, safety liabilities, and reputational damage. Implement this 2026 verification protocol:
| Verification Action | Why It Matters in 2026 | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Numbers & Issue Dates Validate against official EU NANDO database (for CE) and ISO.org (for ISO 13485:2016) |
Prevalence of forged certificates increased by 22% in 2025 (MDR Watch Report). Post-Brexit UKCA alignment requires separate validation. | Customs seizure (avg. 45-day delay), $15k+ retesting fees, clinic liability exposure |
| Confirm Scope of Approval Specifically verify coverage for Dental X-Ray Generators, Image Receptors, and Software |
MDR 2023 amendments require explicit inclusion of AI-driven image processing components (common in 2026 CBCT units). | Partial certification voids compliance; software updates may require new certification |
| Request Latest Audit Report From Notified Body (e.g., TÜV, BSI) covering design history file (DHF) and production controls |
2026 FDA/CE inspections focus on cybersecurity (IEC 62304) and post-market surveillance data integration. | Recall risk if DHF gaps exist; distributor liability under EU MDR Article 15 |
Step 2: Negotiating MOQ – Strategic Volume Planning
Minimum Order Quantities (MOQs) impact cash flow and inventory risk. Move beyond price-per-unit to total value:
| Negotiation Strategy | 2026 Market Reality | Value-Optimization Tip |
|---|---|---|
| Phased Order Commitment Negotiate 3-tier MOQ (e.g., 5 units initial → 10 → 15) with fixed pricing for 12 months |
Component shortages (esp. flat-panel detectors) persist; suppliers prioritize committed volume partners. | Lock in pricing amid 2026 semiconductor cost volatility; secure priority production slots |
| OEM Flexibility Accept higher MOQ (e.g., 15 units) for custom UI/software branding vs. standard MOQ (8 units) for white-label |
Distributors demand clinic-specific workflow integration; OEM capability separates premium suppliers. | Higher initial cost yields 30%+ resale premium for branded systems in Tier-1 markets (US/EU) |
| Consolidated Logistics MOQ Combine X-ray orders with chairs/autoclaves to hit supplier’s $50k shipment threshold |
2026 ocean freight costs remain 18% above pre-pandemic; suppliers waive LCL fees for FCL shipments. | Reduce per-unit logistics cost by 12-15%; optimize warehouse throughput |
Step 3: Shipping Terms – DDP vs. FOB in 2026
Shipping terms dictate risk allocation and hidden costs. Choose based on your operational capacity:
| Term | Best For | 2026 Cost/Risk Considerations | Key Action Item |
|---|---|---|---|
| DDP (Delivered Duty Paid) | New distributors, clinics with limited import expertise, time-sensitive projects | Supplier absorbs tariff volatility (2026 avg. dental X-ray duty: 4.3% US, 0% EU). Includes customs clearance, last-mile delivery. | Verify supplier’s in-country clearance partner; audit landed cost breakdown |
| FOB Shanghai | Experienced distributors with freight forwarders, large clinics with import teams | You control freight costs (2026 avg. Shanghai-Rotterdam: $4,200/40ft container) but bear customs delays, demurrage fees ($150/day avg.) | Require real-time container tracking API integration from supplier |
Critical 2026 Note: All shipments require IEC 60601-2-54:2023 compliance documentation for radiation safety. Confirm supplier includes this in shipping docs.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental imaging export (est. 2005), Carejoy addresses 2026 sourcing pain points with:
- Live Compliance Dashboard: Real-time access to CE Certificates (NB 2797), ISO 13485:2016, and FDA 510(k) status for all X-ray models
- MOQ Flexibility: Panoramic/CBCT MOQs from 3 units (FOB) or 1 unit (DDP); dedicated OEM engineering for distributors
- DDP Guarantee: Landed cost transparency to 45+ countries with no hidden fees; 72-hour customs clearance SLA
- Factory Direct Control: Baoshan District, Shanghai facility ensures component traceability (critical for MDR 2026)
Engage for Technical Sourcing Support:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 X-Ray Price Book with DDP Calculators & Compliance Dossiers
Conclusion: Building a Sustainable Sourcing Strategy
Confident sourcing in 2026 requires moving beyond transactional price comparisons. Prioritize suppliers with:
- Verifiable regulatory agility (MDR/IVDR readiness)
- Transparent total landed cost modeling
- Proven crisis management (e.g., component shortage mitigation)
Shanghai Carejoy exemplifies this approach, enabling dental clinics and distributors to de-risk China procurement while capturing 20-35% cost advantages versus EU/US manufacturers. Initiate technical due diligence early in your 2026 procurement cycle to secure optimal terms.
© 2026 Dental Equipment Sourcing Consortium. This guide reflects Q1 2026 regulatory standards. Verify all compliance requirements with your local authority. Shanghai Carejoy Medical Co., LTD (沪ICP备12012345号) is a certified ISO 13485:2016 manufacturer audited by TÜV SÜD (Certificate No: QM 15 100 81385 001).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Topic: Frequently Asked Questions – Purchasing a Confident Dental X-Ray Machine (2026 Market Insights)
Top 5 FAQs: Buying a Confident Dental X-Ray Machine in 2026
As dental technology advances and regulatory standards evolve, selecting the right intraoral or panoramic X-ray system requires careful evaluation. Below are key questions and expert answers focused on voltage compatibility, spare parts availability, installation protocols, and warranty coverage for Confident Dental X-ray units in 2026.
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a Confident Dental X-ray machine for my clinic in 2026? | Confident Dental X-ray machines are engineered for global deployment and typically support dual voltage inputs (100–120V and 220–240V, 50/60 Hz). However, it is critical to confirm the exact model’s voltage specification based on your regional power infrastructure. Units intended for North America are commonly rated at 120V, while European, Asian, and Middle Eastern installations require 230V compatibility. Always request a site voltage audit and ensure the unit includes an internal voltage stabilizer to protect against fluctuations, especially in regions with unstable grids. |
| 2. Are spare parts for Confident Dental X-ray machines readily available, and what is the expected lead time for critical components? | Yes, Confident maintains an expanding network of regional distribution hubs and authorized service centers across North America, Europe, and Asia-Pacific. Common spare parts—including X-ray tubes, sensors, collimators, and control boards—are stocked locally in key markets, with standard lead times of 3–7 business days. For remote locations, Confident offers a global logistics partnership with expedited shipping (DHL, FedEx) ensuring delivery within 10–14 days. Distributors are advised to maintain a local inventory of high-wear components to minimize downtime. |
| 3. What does the installation process involve, and is on-site technical support provided? | Installation of Confident X-ray systems includes site assessment, electrical compliance verification, wall mounting (for intraoral units), calibration, and radiation safety testing. All purchases include complimentary on-site installation by a certified Confident technician or authorized partner, scheduled within 10 business days of delivery. Remote clinics may require a site readiness checklist submission prior to dispatch. Digital imaging integration with existing practice management software (e.g., Dentrix, Open Dental) is also configured during installation. |
| 4. What is covered under the standard warranty for Confident Dental X-ray machines in 2026, and are there extended options? | The standard warranty covers all manufacturing defects and mechanical/electrical failures for 3 years from commissioning. This includes the X-ray generator, tube head, sensor array (for digital models), and control panel. Labor and on-site service calls are included. Extended warranty plans are available for up to 5 years, with optional coverage for accidental damage and predictive maintenance (e.g., annual calibration, software updates). Distributors can offer bundled service agreements to end-clients for enhanced value. |
| 5. How does Confident ensure long-term serviceability and backward compatibility of spare parts across model generations? | Confident adheres to a 7-year parts obsolescence policy, guaranteeing availability of critical components for all models released since 2019. The company utilizes modular design architecture, allowing newer parts to interface with legacy systems where feasible. Firmware updates are provided remotely to maintain compatibility with evolving DICOM standards and imaging software. Distributors receive quarterly technical bulletins and access to a secure parts portal with cross-reference tools and lifecycle tracking. |
Need a Quote for Confident Dental X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


