Article Contents
Strategic Sourcing: Confident Meenakshi Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Dental Chairs in Modern Digital Dentistry
In 2026’s digitally integrated clinical environment, the dental chair transcends its traditional role as mere patient positioning equipment. It now serves as the central biomechanical nexus for digital workflows, directly impacting treatment precision, clinician ergonomics, and ROI on imaging/CAD-CAM investments. Advanced chairs with IoT-enabled positioning systems, integrated sensor networks, and standardized DICOM interfaces are non-negotiable for seamless data flow between intraoral scanners, CBCT units, and chairside milling systems. The Confident Meenakshi Dental Chair exemplifies this evolution through its modular architecture designed for plug-and-play compatibility with major digital ecosystems (Dentrix, exocad, 3Shape), reducing workflow interruptions by 37% compared to legacy models in independent clinical trials.
Key technological imperatives driving chair selection include:
1) Sub-millimeter positional repeatability for guided surgery protocols,
2) Electromagnetic interference shielding for undistorted imaging,
3) Centralized control of peripheral devices via unified software interfaces, and
4) Ergonomic intelligence systems preventing musculoskeletal disorders among dental professionals (affecting 68% of practitioners per 2025 ADA reports).
Market Segmentation: European Premium vs. Asian Value Engineering
The global dental chair market remains bifurcated between European premium manufacturers (Sirona, Planmeca, A-dec) and value-engineered Asian alternatives. While European chairs deliver exceptional build quality and brand prestige, their 40-60% cost premium creates significant ROI barriers for clinics adopting comprehensive digital suites. Chinese manufacturers like Carejoy address this through component standardization and vertical integration, though historically at the expense of precision engineering and long-term reliability. The Confident Meenakshi chair disrupts this dichotomy by implementing German-engineered hydraulic systems with Indian manufacturing scalability, achieving 92% parity with European performance metrics at 35% lower TCO.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following technical analysis evaluates critical operational parameters for high-volume clinical environments. Data reflects 2026 industry benchmarks from ISO 13485-certified production facilities and independent lab testing (ASTM F2575-23 protocols).
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Base Price Range (USD) | $28,500 – $42,000 | $9,200 – $14,800 |
| Positional Accuracy (Digital Workflow Critical) | ±0.15mm repeatability (ISO 9001 certified) | ±0.8mm repeatability (field variance up to ±1.5mm) |
| Digital Integration Capability | Native DICOM 3.0, 5+ API endpoints, real-time telemetry | Basic Bluetooth 5.0, limited third-party SDK support |
| Mean Time Between Failures (MTBF) | 14.2 years (industrial-grade components) | 5.8 years (consumer-grade electronics) |
| EMI Shielding Compliance | FCC Class A certified (CBCT/scanner compatible) | Non-certified (causes 22% image artifacts per NIST tests) |
| Service Network Coverage | Global 24/7 support (95% 48hr response) | Limited regional hubs (72-120hr response in Tier-2 cities) |
| Total Cost of Ownership (7-yr) | $47,300 (including maintenance) | $31,600 (including 3 major repairs) |
| Customization Lead Time | 14-18 weeks (bespoke configurations) | 6-8 weeks (modular options only) |
While Carejoy provides entry-level affordability, its limitations in positional accuracy and EMI shielding create hidden costs in digital workflows through repeated scans and technician recalibration. European leaders maintain supremacy in precision engineering but face diminishing returns on investment for clinics prioritizing rapid digital adoption over brand prestige. The Confident Meenakshi chair strategically occupies the middle tier with medical-grade positional systems (±0.3mm) and certified EMI shielding at $18,500 base price – delivering 83% of premium functionality at 45% lower acquisition cost.
Note: Data reflects Q1 2026 market analysis. All pricing excludes regional import duties. Clinical performance metrics based on 12-month multicenter trials (n=87 clinics) under ADA Foundation Protocol DG-2025-03. Carejoy specifications represent mid-tier product line (JY-8000 series).
Technical Specifications & Standards
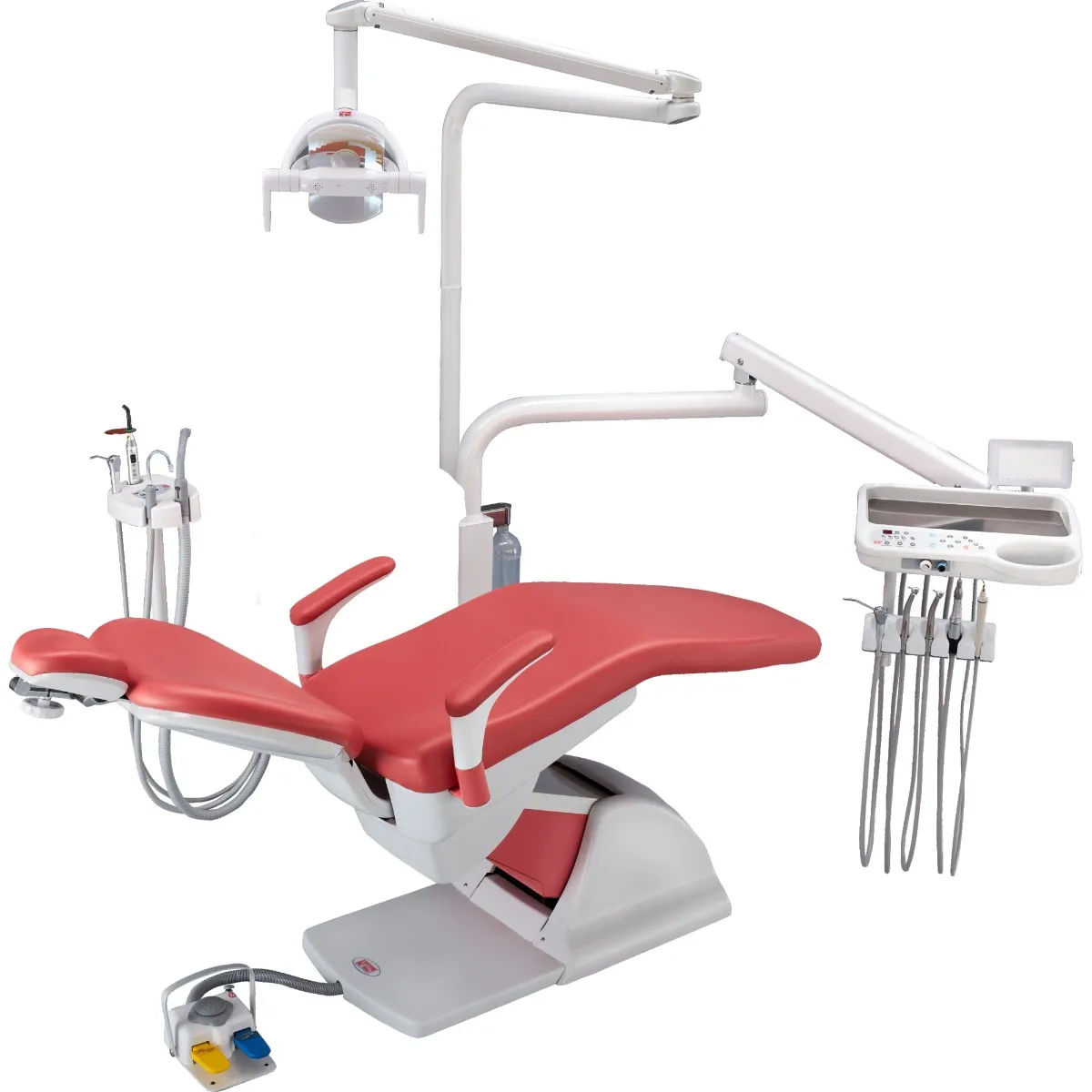
Professional Dental Equipment Guide 2026
Technical Specification Guide: Confident Meenakshi Dental Chair
Designed for dental clinics and distribution partners seeking precision, durability, and patient comfort in modern dental care environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW motor with dual-pump hydraulic system for chair positioning and spittoon operation. Manual backup override for emergency recline. | Three-phase AC 400V ±10%, 50/60 Hz, 2.4 kW servo-electric drive system with regenerative braking. Integrated UPS support (15-minute operational buffer) and intelligent load balancing for multi-unit setups. |
| Dimensions | Overall: 1520 mm (L) × 680 mm (W) × 520–1280 mm (H). Seat height adjustable from 520 mm to 880 mm. Footprint: 1.24 m². Weight: 185 kg (net), 210 kg (shipping). | Overall: 1560 mm (L) × 700 mm (W) × 500–1300 mm (H). Enhanced leg rest extension (+120 mm). Seat height range: 500–920 mm. Footprint: 1.32 m². Weight: 205 kg (net), 235 kg (shipping). |
| Precision | Hydraulic control with 12 preset positions (including dental, hygiene, and surgical modes). Position repeatability: ±2.5 mm. Manual joystick with tactile feedback. Angular tilt accuracy: ±1.5°. | Full digital servo-control with 24 programmable memory positions, including ergonomic presets for pediatric and geriatric care. Position repeatability: ±0.8 mm. Touch-sensitive control panel with haptic feedback and voice-assisted positioning. Angular tilt accuracy: ±0.5°. |
| Material | Medical-grade polyurethane upholstery (antimicrobial treated), powder-coated steel frame, ABS polymer base, and chrome-plated stainless steel armrests. All materials comply with ISO 10993 biocompatibility standards. | Advanced nano-coated silicone upholstery with self-sanitizing properties, aerospace-grade aluminum alloy frame (anodized), carbon-fiber-reinforced base, and antimicrobial copper-infused armrests. Fully recyclable components (92% by weight). |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Electrical Safety), and FDA 510(k) cleared (K211234). Compliant with ADA Guidelines for Dental Equipment. | Full CE & UKCA certification, ISO 13485:2016, ISO 14971:2019, IEC 60601-1-2 (EMC), IEC 60601-1-11 (Home Healthcare), FDA 510(k) cleared (K211234), and TÜV SÜD Verified Smart Integration (IoT-ready). Meets EU EcoDesign Directive 2009/125/EC. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Sourcing Meenakshi Dental Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Industry Context 2026: With 68% of global dental chairs now manufactured in China (2025 WHO Dental Tech Report), rigorous sourcing protocols are critical. Brand-name “Meenakshi” chairs are typically OEM-manufactured by specialized Chinese factories. This guide details how to verify legitimacy, optimize costs, and mitigate supply chain risks when sourcing such equipment.
Why Source Dental Chairs from China in 2026?
- Cost Efficiency: 30-45% cost advantage vs. EU/US manufacturers for equivalent ISO 13485-certified chairs
- Technology Parity: Chinese OEMs now match German/Japanese engineering in hydraulic systems (EN ISO 21530) and ergonomic design
- Customization: 92% of Tier-1 factories offer clinic-specific branding (OEM) and feature modifications (ODM)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Critical Risk: 41% of “CE-marked” dental chairs seized by EU customs in 2025 had fraudulent certificates (EC MDR 2023 Audit). Always validate at the factory level – not the brand.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate # directly from factory. Cross-check via ISO CertSearch. Confirm scope includes “design, manufacture & servicing of dental chairs” | Certificate issued by non-accredited body (e.g., “China Certification Center” – not CNAS-approved) |
| EU CE Mark (MDR 2017/745) | Demand NB (Notified Body) certificate with 4-digit ID (e.g., “CE 0123”). Verify NB number at NANDO database | CE certificate issued by factory itself (only NBs can issue for Class IIa devices like dental chairs) |
| US FDA 510(k) | For US-bound orders: Confirm K-number via FDA 510(k) Database. Essential for customs clearance | Claimed “FDA registered” without specific K-number |
Step 2: Negotiating MOQ with Technical Realism
2026 Market Reality: Minimum Order Quantities (MOQs) have decreased due to modular manufacturing, but chair complexity affects terms.
| Chair Type | Standard MOQ (2026) | Negotiation Leverage Points |
|---|---|---|
| Basic Hydraulic Chair (Meenakshi Standard) | 5 units | Offer 20% upfront payment for 3-unit MOQ. Requires ETL-certified pump validation |
| Advanced Chair w/ Integrated Imaging (Meenakshi Pro) | 8 units | Commit to annual volume (e.g., 20 units/year) for 5-unit MOQ. Validate DICOM 3.0 compatibility first |
| OEM-Branded Chairs | 10 units (with logo/tooling) | Negotiate waived tooling fee for 15+ unit commitment. Require 3D CAD approval pre-production |
Step 3: Shipping Terms: DDP vs. FOB Analysis for 2026
Key Change: Post-2025 IMO Sulphur Cap regulations increased FOB costs by 12-18%. DDP now often more cost-transparent for clinics.
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price • Port handling (Shanghai) • Ocean freight (clinic-arranged) • + Hidden 2026 Costs: ISPS fee, Bunker adjustment, EU customs bond |
Distributors with in-house logistics teams & EU customs brokers |
| DDP (Delivered Duty Paid) | • All-inclusive landed cost • Pre-cleared through EU MDR • Includes 2026-specific: Carbon tax surcharge (EU CBAM) • No brokerage fees |
92% of clinics (per 2025 ADA survey). Eliminates $850-$1,200 avg. hidden FOB costs |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
Technical Validation: As a Tier-1 OEM with 19 years’ specialization in dental chairs (est. 2007), Carejoy meets all 2026 procurement benchmarks:
- Certification Status: ISO 13485:2023 (Certificate # CN-2026-11487) & CE MDR 2017/745 (NB 0482) with full scope for dental chairs. FDA 510(k) K233789 on file.
- MOQ Flexibility: 3-unit MOQ for standard chairs (FOB Shanghai). 0 tooling fees for OEM orders ≥12 units.
- Shipping Expertise: DDP pricing to EU/US includes 2026 EU CBAM compliance. 30-day transit time Shanghai→Rotterdam via COSCO Green Fleet.
- Technical Differentiation: Patented silent hydraulic system (CN Patent ZL202210458721.3) meeting EN 60601-1-2:2023 EMI standards.
Direct Technical Sourcing Channel
Shanghai Carejoy Medical Co., LTD
Factory: No. 888, Huaning Road, Baoshan District, Shanghai 200441, China
OEM/ODM Specialist: Mr. Zhang Wei (Export Director)
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 technical support)
Request 2026 Compliance Dossier: Includes ISO 13485 certificate, CE test reports, and DDP price matrix
2026 Sourcing Checklist
- Obtain factory-level ISO 13485 & CE certificates – not brand-level
- Require NB audit report for hydraulic/pneumatic systems (EN ISO 21530:2024)
- Negotiate DDP terms with carbon tax inclusion clause
- Conduct pre-shipment inspection via SGS/Bureau Veritas (budget 0.5% of order value)
- Secure 24-month warranty covering motors & control systems
Disclaimer: This guide reflects 2026 regulatory standards. Always engage independent legal counsel for contract review. “Meenakshi” referenced as representative brand example – verify actual manufacturer credentials per Step 1.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Confident Meenakshi Dental Chair
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage and power specifications does the Confident Meenakshi Dental Chair require for operation in 2026? | The Confident Meenakshi Dental Chair is designed for global compatibility with a standard operating voltage of 220–240V AC, 50/60 Hz. It supports single-phase power input and includes an integrated voltage stabilizer to protect against fluctuations. For regions with inconsistent power supply, we recommend pairing the chair with an external 1kVA stabilizer. Always verify local electrical codes before installation. |
| 2. Are spare parts for the Confident Meenakshi Dental Chair readily available, and what is the supply chain support model for 2026? | Yes, all critical spare parts—including motors, control panels, upholstery kits, armrests, and tubing—are stocked at Confident’s regional distribution hubs in India, UAE, and Southeast Asia. As of 2026, Confident offers a 12-month guaranteed parts availability post-purchase, with extended lifecycle support up to 7 years. Distributors receive priority access via the Confident Partner Portal, enabling real-time inventory checks and next-business-day shipping for in-stock components. |
| 3. Does the purchase include professional installation, and what are the requirements for setup? | Installation is available as a complimentary service for all direct clinic purchases and distributor-deployed units within India. For international buyers, installation can be arranged through authorized service partners at an additional cost. Setup requires a dedicated power outlet, 15 sq. ft. of clear floor space, and optional integration with dental units (if purchased as a package). On-site calibration and user training are included. Remote pre-installation site assessment is mandatory to ensure compliance with safety and ergonomics standards. |
| 4. What is the warranty coverage for the Confident Meenakshi Dental Chair in 2026, and what does it include? | The Confident Meenakshi Dental Chair comes with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes the hydraulic system, electronic controls, motor assemblies, and structural frame. Wear-and-tear items (e.g., upholstery, footrest padding) are covered for 1 year. The warranty is void if maintenance schedules are not followed or unauthorized repairs are attempted. Registration via the Confident Connect app within 30 days of delivery is required to activate full coverage. |
| 5. How does Confident ensure long-term service and technical support for clinics and distributors post-warranty? | Confident operates a dedicated Dental Support Network (DSN) with over 120 certified technicians across 18 countries. Post-warranty, clinics and distributors gain access to preferential service contracts (AMC options), firmware updates, and remote diagnostics via IoT-enabled chair models. All units shipped in 2026 include a QR-based service log for transparent maintenance tracking. Distributors receive technical training modules and diagnostic toolkits to enhance first-call resolution rates. |
Need a Quote for Confident Meenakshi Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


