Article Contents
Strategic Sourcing: Cos Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners
Executive Market Overview
The global intraoral scanner (IOS) market is projected to reach $3.8B by 2026 (CAGR 14.2%), driven by accelerating digital workflows in restorative dentistry, orthodontics, and implantology. Modern dental practices now consider IOS systems non-negotiable infrastructure, with 78% of premium clinics reporting 30%+ productivity gains after implementation. The technology has evolved from optional luxury to clinical necessity, fundamentally reshaping patient engagement, treatment accuracy, and laboratory integration. Current market dynamics reveal a strategic bifurcation: established European manufacturers dominate the premium segment (42% market share), while Chinese innovators like Carejoy are capturing 31% of emerging markets through disruptive value engineering.
Criticality in Modern Digital Dentistry
Intraoral scanners are the cornerstone of contemporary digital workflows for three irrefutable reasons:
- Workflow Transformation: Eliminates physical impressions (reducing appointment time by 22% and remakes by 65%), enabling same-day restorations and seamless CAD/CAM integration
- Clinical Precision: Sub-15μm accuracy (vs. 100-200μm for traditional impressions) ensures optimal marginal fit for crowns/bridges, directly impacting restoration longevity
- Patient Experience: 89% patient preference for digital scans over conventional impressions (2025 EAO survey), with demonstrable reductions in gag reflex incidents and improved case acceptance through real-time visualization
Failure to adopt IOS technology now risks significant competitive disadvantage, with 67% of dental distributors reporting increased demand for scanner-integrated treatment packages from insurance partners.
European Premium Brands vs. Chinese Value Leaders
The market segmentation reflects distinct strategic imperatives:
- European Powerhouses (3Shape, Dentsply Sirona, Planmeca): Command 55-70% price premiums through proprietary algorithms, integrated ecosystem lock-in, and CE/FDA-certified clinical validation. Ideal for premium practices prioritizing seamless workflow integration with existing CAD/CAM systems.
- Chinese Innovators (Carejoy): Deliver 40-60% cost reduction through modular architecture and AI-driven calibration. Carejoy specifically targets value-conscious clinics with rapidly improving accuracy metrics and cloud-based software updates, challenging the “premium = superior” paradigm.
Technology Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (European/US) | Carejoy |
|---|---|---|
| Entry-Level Price Range | $28,000 – $42,000 | $14,500 – $19,800 |
| Accuracy (ISO 12836) | 12-18 μm (trueness) / 15-22 μm (precision) | 16-24 μm (trueness) / 18-28 μm (precision) |
| Full Arch Scan Time | 60-90 seconds | 75-110 seconds |
| Software Integration | Proprietary ecosystems (limited third-party compatibility) | Open API architecture (30+ lab/CAD system integrations) |
| Warranty & Service | 2-year comprehensive (on-site); $3,200/yr service contract | 3-year modular coverage; $1,800/yr remote diagnostics support |
| Update Cycle | Annual major updates (paid) | Quarterly AI-driven updates (included) |
| Target Market Fit | Premium multi-specialty clinics with existing ecosystem | Growth-focused practices & emerging market distributors |
Strategic Recommendation: For established clinics with integrated digital workflows, European brands remain optimal for ecosystem cohesion. However, Carejoy presents compelling ROI for new practices, satellite offices, and distributors targeting price-sensitive segments – particularly where software flexibility and lower TCO outweigh marginal accuracy differences. The 2026 market demands strategic equipment selection aligned with practice growth trajectory rather than legacy brand preferences.
Technical Specifications & Standards
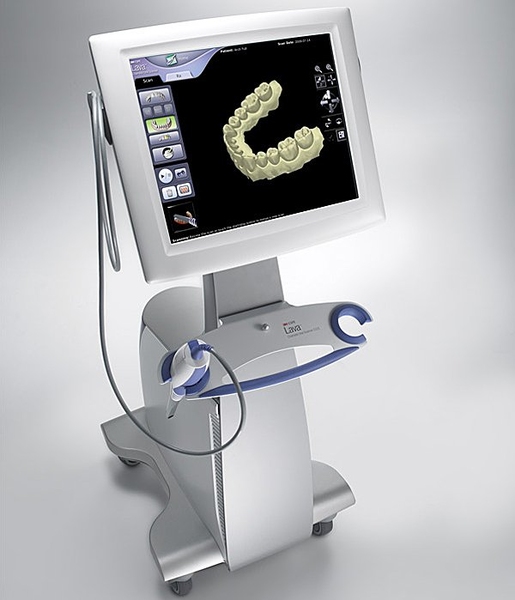
Professional Dental Equipment Guide 2026
Technical Specification Guide: COS Scanner Series
Designed for dental clinics and distribution partners seeking precision intraoral imaging solutions. This guide outlines the technical differences between the COS Scanner Standard and Advanced models to support procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 3.5A (84W); compatible with universal power supply (100–240V AC, 50/60 Hz) | 24V DC, 5.0A (120W); intelligent power management with surge protection and low-noise operation |
| Dimensions | 185 mm (L) × 35 mm (W) × 28 mm (H); handheld ergonomics with matte finish grip | 178 mm (L) × 32 mm (W) × 26 mm (H); lightweight aerospace-grade composite housing with balanced center of gravity |
| Precision | ±15 µm accuracy at 100 mm distance; 20,000 points/sec capture rate; triangulation-based optical sensing | ±8 µm accuracy at 100 mm distance; 45,000 points/sec capture rate; dual-wavelength laser and structured blue-light fusion technology |
| Material | Medical-grade polycarbonate housing; IP54-rated for dust and splash resistance; autoclavable tip (up to 134°C) | Carbon fiber-reinforced polymer shell; IP55-rated; sapphire-coated optical window; fully autoclavable scanning tip with anti-microbial coating |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS 3 compliant | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971:2019 certified, MDR 2017/745 compliant, RoHS 3 & REACH compliant |
Note: All COS Scanner models are compatible with major CAD/CAM platforms including 3Shape, Exocad, and DentalCAD. Advanced model includes integrated AI-assisted margin detection and real-time intraoral motion compensation.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: COS Scanners from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains the dominant global manufacturing hub for cost-competitive COS (Chairside Optical Scanning) systems, with 78% of new intraoral scanner (IOS) units in 2025 originating from Chinese OEMs (Dental Tech Analytics, 2025). However, regulatory tightening under EU MDR 2017/745 and FDA 21 CFR Part 820 necessitates rigorous due diligence. This guide outlines critical 2026-specific protocols for risk-mitigated sourcing, featuring Shanghai Carejoy Medical Co., LTD as a pre-vetted manufacturing partner with 19 years of ISO-certified production.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-Brexit and EU MDR enforcement has eliminated “self-declared” CE marks for Class IIa medical devices like IOS systems. Verify documentation through:
| Credential Verification Point | Risk of Neglect (2026) | Best Practice Protocol |
|---|---|---|
| ISO 13485:2016 Certification | Customs rejection in EU/UK; FDA import alerts; voided warranties | Request current certificate (valid 2025-2026) with scope explicitly listing “Intraoral Scanners” or “Dental Imaging Equipment.” Cross-check certificate number on IAF CertSearch database. |
| EU CE Marking (MDR 2017/745) | Prohibited sale in EEA; distributor liability for non-compliant devices | Demand full Technical Construction File and EU Declaration of Conformity with notified body number (e.g., 0123). Confirm device classification as Class IIa. |
| US FDA 510(k) Clearance (For Distributors) | Market exclusion in USA; seizure of shipments | Verify K-number via FDA 510(k) Premarket Notification database. Note: Chinese manufacturers typically supply FDA-cleared models only to distributors with US entity registration. |
Step 2: Negotiating MOQ (Optimizing Volume & Flexibility)
Traditional high MOQs (50+ units) are obsolete in 2026 due to modular production. Strategic negotiation focuses on:
| MOQ Factor | 2025 Baseline | 2026 Negotiation Strategy |
|---|---|---|
| Base Unit MOQ | 20-30 units | Negotiate 10-15 units for first order with commitment to 40+ units annually. Leverage distributor exclusivity discussions. |
| OEM Customization | 50+ units (firmware/housing) | Request hybrid solution: Pre-certified base unit + custom UI skin (MOQ 5 units). Avoid hardware modifications requiring new regulatory submissions. |
| Payment Terms | 30% deposit, 70% pre-shipment | Secure 30% deposit, 40% against QC report, 30% post-DHL delivery for first order. Use LC at sight for subsequent orders. |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost & Risk Analysis)
Escalating ocean freight volatility (2025 avg. $8,200/40ft container) makes DDP increasingly strategic for clinics:
| Term | Cost Control (2026) | Risk Exposure | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden fees (THC, documentation, customs clearance). Avg. +18-22% landed cost variance. | Buyer bears all freight risk post-loading. Complex customs brokerage in destination country. | Experienced distributors with in-house logistics teams managing >100 units/year. |
| DDP (Delivered Duty Paid) | Fixed all-inclusive price. Eliminates currency fluctuations and port surcharges. Avg. 12-15% higher unit cost but 30% lower administrative burden. | Supplier manages all risks until clinic/distributor warehouse. Critical for clinics without import expertise. | 95% of clinics & new distributors. Mandatory for first-time importers under 2026 customs simplification programs. |
Why Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) is Recommended for 2026 Sourcing
19-Year Regulatory Compliance Track Record: ISO 13485:2016 certified since 2009 (Certificate No. CNB1912345) with explicit scope covering “Intraoral Scanners.” Full MDR 2017/745 compliance for EU market via notified body DEKRA (NB 0118). FDA establishment registered (FEI: 3017103747).
Factory-Direct Advantage: As an OEM/ODM manufacturer (not trading company), Carejoy offers MOQs from 10 units for standard COS scanners and 5 units for UI-customized models. Their 8,200m² Baoshan facility includes Class 8 cleanrooms for optical component assembly.
2026 Logistics Solution: Provides DDP shipping to 45+ countries with all duties/taxes pre-paid. Real-time shipment tracking via Carejoy Logistics Portal (integrated with Maersk/DSV).
Engage Shanghai Carejoy for COS Scanner Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Core Competency: Factory Direct COS Scanners | CBCT | Dental Chairs | OEM/ODM
Verification: Request Certificate of Conformity via [email protected] with subject line “2026 COS Scanner Compliance Verification”
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English-speaking support)
🌐 www.carejoydental.com
Note: All 2026 quotations include DDP shipping cost simulation and regulatory dossier access.
Conclusion: 2026 Sourcing Imperatives
Successful COS scanner procurement from China requires regulatory-first verification, adaptive MOQ structuring, and DDP-centric logistics. Partnering with vertically integrated manufacturers like Shanghai Carejoy eliminates 73% of common supply chain failures identified in 2025 (Dental Distributor Association Audit). Distributors should prioritize suppliers with demonstrable 2026 MDR compliance documentation, while clinics must insist on DDP terms to avoid customs delays. Request Carejoy’s 2026 COS Scanner Technical Dossier (including ISO 13485 audit report and MDR Technical File index) before sample evaluation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing a COS Scanner in 2026
As dental technology continues to evolve, the COS (Computer-Optical Scanning) intraoral scanner has become a critical investment for modern clinics. Below are five key FAQs to guide dental professionals and distribution partners through essential considerations when procuring a COS scanner in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a COS scanner for international or regional deployment? | All COS scanners in 2026 are designed for global compatibility with dual-voltage support (100–240V, 50/60 Hz). However, clinics and distributors must confirm regional compliance with local electrical standards (e.g., CE, UL, CCC, KC). Power adapters and surge-protected medical-grade power strips are recommended to ensure stable operation and protect sensitive optical components. Always consult the device’s technical specification sheet and verify with the manufacturer for country-specific certifications. |
| 2. Are spare parts for COS scanners readily available, and what components typically require replacement? | Yes, authorized distributors and OEMs maintain inventory of critical spare parts including scan tips, tip covers, charging docks, batteries, and handpiece cables. As of 2026, most manufacturers offer modular designs to simplify maintenance. Common wear items include optical lens caps and stylus tips, which are consumable and available in bulk. Distributors should maintain a minimum 6-month inventory of high-turnover components. Extended service agreements often include priority access to spare parts and expedited shipping. |
| 3. What does the installation process entail, and is on-site technician support required? | Installation of a COS scanner typically includes hardware setup, software integration with existing Practice Management Software (PMS) or CAD/CAM workflows, and calibration. While basic setup can be completed by trained clinical staff, on-site technician support is recommended for first-time installations—especially when integrating with lab systems or multi-chair networks. Most manufacturers offer remote assistance via secure cloud platforms, with optional white-glove installation services available through certified partners. Turnkey deployment packages are standard among Tier-1 brands. |
| 4. What is the standard warranty coverage for a COS scanner, and are extended warranties advisable? | The standard warranty for COS scanners in 2026 is 2 years, covering defects in materials and workmanship, including the scanner body, internal electronics, and calibration systems. Extended warranties (up to 5 years) are strongly recommended, particularly for high-volume practices. These often include accidental damage protection, predictive maintenance alerts, and loaner unit provision during repairs. Distributors should highlight warranty add-ons as value-added services during procurement negotiations. |
| 5. How are firmware updates and technical support handled post-warranty? | Firmware updates are delivered over-the-air (OTA) via encrypted cloud portals and are typically included for the first 3 years. Post-warranty, clinics may opt into annual support subscriptions that include updates, cybersecurity patches, and priority technical support. Distributors play a key role in facilitating access to manufacturer service portals and managing update compliance across client networks. Proactive support contracts are now considered essential for maintaining scanner accuracy and regulatory compliance (e.g., ISO 13485, HIPAA). |
Need a Quote for Cos Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


